Methylsilane
From Wikipedia, the free encyclopedia
| Methylsilane | |
|---|---|
 |
 |
| Identifiers | |
| CAS number | 992-94-9 |
| ChemSpider | 63613 |
| Jmol-3D images | {{#if:C[SiH3]|Image 1 |
| |
| Properties | |
| Molecular formula | CH6Si |
| Molar mass | 46.14 g/mol |
| Appearance | Colorless hygroscopic gas[1] |
| Density | 0.628 g cm-3 |
| Melting point | −157 °C; −251 °F; 116 K |
| Boiling point | −57 °C; −71 °F; 216 K |
| Solubility in water | Hydrolyzes[1] |
| Hazards | |
| EU classification | |
| R-phrases | R12, R14, R20/21 |
| S-phrases | S16, S24, S26, S36/37/39 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
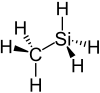
Methylsilane (CH3-SiH3) is a compound of silicon, carbon, and hydrogen. The reaction of methylsilane and Si+ produces several active organosilicon species.[2]
References
- ↑ 1.0 1.1 MSDS from Matheson Tri-Gas
- ↑ Nguyen, Kiet A.; Gordon, Mark S.; Raghavachari, Krishnan (1994). "Mechanisms and Energetics of the Reaction of Si+ with CH3-SiH3". The Journal of Physical Chemistry 98 (27): 6704. doi:10.1021/j100078a010.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.