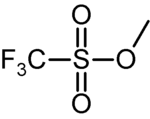
Methyl trifluoromethansulfonate
| Methyl triflate | |
|---|---|
 | |
 | |
| IUPAC name Methyl trifluoromethanesulfonate | |
| Other names Trifluoromethanesulfonic acid, methyl ester | |
| Identifiers | |
| CAS number | 333-27-7 |
| PubChem | 9526 |
| ChemSpider | 9153 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C2H3F3O3S |
| Molar mass | 164.10 g/mol |
| Appearance | Colourless Liquid |
| Density | 1.496 g/mL |
| Melting point | −64 °C; −83 °F; 209 K |
| Boiling point | 100 °C; 212 °F; 373 K |
| Solubility in water | Hydrolyzes |
| Hazards | |
| R-phrases | R10, R34 |
| S-phrases | S26, S36/37/39, S45 |
| Main hazards | Corrosive |
| Flash point | 38 °C; 100 °F; 311 K |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Methyl trifluoromethanesulfonate, commonly abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid that is a very powerful (and very dangerous) methylating reagent in chemistry.[1]
Reactivity
This compound is closely related to methyl fluorosulfonate (FSO2OCH3), which is an older and less common reagent. These compounds alkylate faster and with wider range of substrates than traditional methylating agents such as methyl iodide. One ranking of alkylating agents is (CH3)3O+ > CF3SO2OCH3 ~ FSO2OCH3 > (CH3)2SO4> CH3I. It will alkylate many functional groups that are only weakly basic such as aldehydes, amides, and nitriles. It does not methylate benzene or the bulky 2,6-di-tert-butylpyridine.[1] Its ability to methylate N-heterocycles is exploited in certain deprotection schemes.[2]
See also
- Triflate
- Magic methyl
References
- ↑ 1.0 1.1 Roger W. Alder, Justin G. E. Phillips, Lijun Huang, Xuefei Huang, "Methyltrifluoromethanesulfonate" Encyclopedia of Reagents for Organic Synthesis 2005 John Wiley & Sons, doi:10.1002/047084289X.rm266m.pub2
- ↑ Albert I. Meyers and Mark E. Flanagan. (1998), "2,2'-Dimethoxy-6-formylbiphenyl", Org. Synth.; Coll. Vol. 9: 258