Methocarbamol
 | |
|---|---|
| Systematic (IUPAC) name | |
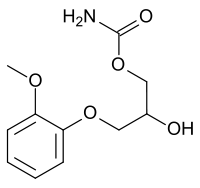
| (RS)-2-hydroxy-3-(2-methoxyphenoxy)propyl carbamate | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682579 |
| Pregnancy cat. | x |
| Legal status | ℞-only (US) OTC(Canada) |
| Routes | Oral, intravenous |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Half-life | 1.14–1.24 hours[1] |
| Identifiers | |
| CAS number | 532-03-6 |
| ATC code | M03BA03 |
| PubChem | CID 4107 |
| DrugBank | DB00423 |
| ChemSpider | 3964 |
| UNII | 125OD7737X |
| KEGG | D00402 |
| ChEMBL | CHEMBL1201117 |
| Chemical data | |
| Formula | C11H15NO5 |
| Mol. mass | 241.241 g/mol |
| SMILES
| |
| |
| | |
Methocarbamol is a central muscle relaxant used to treat skeletal muscle spasms. Under the trade name Robaxin, it is marketed by Actient Pharmaceuticals in the United States and Pfizer in Canada.
Metabolism
Methocarbamol is the carbamate of guaifenesin, but does not produce guaifenesin as a metabolite, because the carbamate bond is not hydrolyzed metabolically; metabolism is by Phase I ring hydroxylation and O-demethylation, followed by Phase II conjugation. All the major metabolites are unhydrolyzed carbamates.[2][3]
Marketing

Methocarbamol is marketed under different names when presented in combination with other active ingredients. In combination with acetaminophen (Paracetamol), under trade names Robaxacet and Tylenol Body Pain Night, whereas Robax Platinum is the trade name for a formulation of methocarbamol and ibuprofen.[4][5] A combination of methocarbamol and aspirin is marketed as Robaxisal, however in Spain Robaxisal is used for the Paracetamol combination instead of Robaxacet.
Abuse potential
Unlike other carbamates such as meprobamate and its prodrug carisoprodol, methocarbamol has greatly reduced abuse potential. Studies comparing it to lorazepam (Ativan) and diphenhydramine (Benadryl), along with placebo, find that methocarbamol produces increased "liking" responses and some sedative-like effects, however, at higher doses dysphoria is reported. It is considered to have an abuse profile similar to, but weaker than, lorazepam.[6]
Side-effects
Potential side-effects include: drowsiness, dizziness, upset stomach, flushing, blurred vision, and fever. Serious side-effects include the development of a severe skin rash or itching, slow heart rate, fainting, jaundice, persistent nausea/vomiting, stomach/abdominal pain, mental/mood changes, clumsiness, trouble urinating, signs of infection. If taken in large amounts at once or more than directed or as prescribed, dysphoria or suicidal thoughts may occur.[7] In addition, methocarbamol may cause urine to turn black, blue, or green. However, this effect is harmless.[8]
Because of potential for side-effects, this drug is considered to be a high-risk medication for the elderly.[9]
Chemistry
Methocarbamol can be synthesized from guaifenesin by successive reaction with phosgene and then ammonia.[10][11]
References
- ↑ Sica DA, Comstock TJ, Davis J, Manning L, Powell R, Melikian A, Wright G. (1990). "Pharmacokinetics and protein binding of methocarbamol in renal insufficiency and normals". European Journal of Clinical Pharmacology 39 (2): 193–4. doi:10.1007/BF00280060. PMID 2253675.
- ↑ Methocarbamol. In: DRUGDEX System [intranet database]. Greenwood Village, Colorado: Thomson Healthcare; c1974–2009 [cited 2009 Feb 10].
- ↑ Bruce RB, Turnbull LB, Newman JH. (1971 Jan). "Metabolism of methocarbamol in the rat, dog, and human". J Pharm Sci 60 (1): 104–106. doi:10.1002/jps.2600600120. PMID 5548215.
- ↑ "New Drugs and Indications Reviewed at the May 2003 DEC Meeting" (PDF). ESI Canada. Retrieved 2008-11-14.
- ↑ "Tylenol Body Pain Night Overview and Dosage" (website). Tylenol Canada. Retrieved 2012-04-23.
- ↑ "Subjective and behavioral effects of diphenhydramine, lorazepam and methocarbamol: evaluation of abuse liability". Journal of Pharmacology and Experimental Therapeutics. Retrieved 2011-05-06.
- ↑ METHOCARBAMOL – ORAL (Robaxin) side effects, medical uses, and drug interactions. Medicinenet.com. Retrieved on 2011-11-09.
- ↑ Methocarbamol: MedlinePlus Drug Information. Nlm.nih.gov. Retrieved on 2011-11-09.
- ↑ See NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly
- ↑ R.S. Murphey, U.S. Patent 2,770,649 (1956)
- ↑ Yale, H. L.; Pribyl, E. J.; Braker, W.; Bergeim, F. H.; Lott, W. A. (1950). Journal of the American Chemical Society 72 (8): 3710. doi:10.1021/ja01164a107.
| |||||||||||||||||||||||||||||||||||||||||||||||||
