Metabolic control analysis
Metabolic control analysis (MCA) is a mathematical framework for describing metabolic, signaling and genetic pathways. MCA quantifies how variables, such as fluxes and species concentrations, depend on network parameters. In particular it is able to describe how network dependent properties, called control coefficients, depend on local properties called elasticities.[1][2][3]
MCA was originally developed to describe the control in metabolic pathways but was subsequently extended to describe signaling and genetic networks. MCA has sometimes also been referred to as Metabolic Control Theory but this terminology was rather strongly opposed by Henrik Kacser, one of the founders[citation needed].
More recent work[4] has shown that MCA can be mapped directly on to classical control theory and are as such equivalent.
Biochemical systems theory[5] is a similar formalism, though with a rather different objectives. Both are evolutions of an earlier theoretical analysis by Joseph Higgins.[6]
Control Coefficients
A control coefficient[7][8][9] measures the relative steady state change in a system variable, e.g. pathway flux (J) or metabolite concentration (S), in response to a relative change in a parameter, e.g. enzyme activity or the steady-state rate ( ) of step i. The two main control coefficients are the flux and concentration control coefficients. Flux control coefficients are defined by:
) of step i. The two main control coefficients are the flux and concentration control coefficients. Flux control coefficients are defined by:

and concentration control coefficients by:

Summation Theorems
The flux control summation theorem was discovered independently by the Kacser/Burns group and the Heinrich/Rapoport group in the early 1970s and late 1960s. The flux control summation theorem implies that metabolic fluxes are systemic properties and that their control is shared by all reactions in the system. When a single reaction changes its control of the flux this is compensated by changes in the control of the same flux by all other reactions.


Elasticity Coefficients
The elasticity coefficient measures the local response of an enzyme or other chemical reaction to changes in its environment. Such changes include factors such as substrates, products or effector concentrations. For further information please refer to the dedicated page at Elasticity Coefficients.
Connectivity Theorems
The connectivity theorems are specific relationships between elasticities and control coefficients. They are useful because they highlight the close relationship between the kinetic properties of individual reactions and the system properties of a pathway. Two basic sets of theorems exists, one for flux and another for concentrations. The concentration connectivity theorems are divided again depending on whether the system species  is different from the local species
is different from the local species  .
.


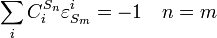
Control Equations
It is possible to combine the summation with the connectivity theorems to obtain closed expressions that relate the control coefficients to the elasticity coefficients. For example, consider the simplest non-trivial pathway:

We assume that  and
and 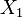 are fixed boundary species so that the pathway can reach a steady state. Let the first step have a rate
are fixed boundary species so that the pathway can reach a steady state. Let the first step have a rate  and the second step
and the second step 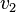 . Focusing on the flux control coefficients, we can write one summation and one connectivity theorem for this simple pathway:
. Focusing on the flux control coefficients, we can write one summation and one connectivity theorem for this simple pathway:
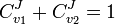

Using these two equations we can solve for the flux control coefficients to yield:


Using these equations we can look at some simple extreme behaviors. For example, let us assume that the first step is completely insensitive to its product (i.e. not reacting with it), S, then  . In this case, the control coefficients reduce to:
. In this case, the control coefficients reduce to:


That is all the control (or sensitivity) is on the first step. This situation represents the classic rate-limiting step that is frequently mentioned in text books. The flux through the pathway is completely dependent on the first step. Under these conditions, no other step in the pathway can affect the flux. The effect is however dependent on the complete insensitivity of the first step to its product. Such a situation is likely to be rare in real pathways. In fact the classic rate limiting step has almost never been observed experimentally. Instead, a range of limitingness is observed, with some steps having more limitingness (control) than others.
We can also derive the concentration control coefficients for the simple two step pathway:


An alternative approach to deriving the control equations is to consider the perturbations explicitly. Consider making a perturbation to  which changes the local rate
which changes the local rate  . The effect on the steady-state to a small change in
. The effect on the steady-state to a small change in  is to increase the flux and concentration of S. We can express these changes locally by describing the change in
is to increase the flux and concentration of S. We can express these changes locally by describing the change in  and
and 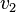 using the expressions:
using the expressions:


The local changes in rates are equal to the global changes in flux, J. In addition if we assume that the enzyme elasticity of  with respect to
with respect to  is unity, then
is unity, then


Dividing both sides by the fractional change in  and taking the limit
and taking the limit  yields:
yields:


From these equations we can choose either to eliminate  or
or  to yield the control equations given earlier. We can do the same kind of analysis for the second step to obtain the flux control coefficient for
to yield the control equations given earlier. We can do the same kind of analysis for the second step to obtain the flux control coefficient for  . Note that we have expressed the control coefficients relative to
. Note that we have expressed the control coefficients relative to  and
and  but if we assume that
but if we assume that  then the control coefficients can be written with respect to
then the control coefficients can be written with respect to  as before.
as before.
Three Step Pathway
Consider the simple three step pathway:

where  and
and 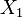 are fixed boundary species, the control equations for this pathway can be derived in a similar manner to the simple two step pathway although it is somewhat more tedious.
are fixed boundary species, the control equations for this pathway can be derived in a similar manner to the simple two step pathway although it is somewhat more tedious.



where D the denominator is given by:

Note that every term in the numerator appears in the denominator, this ensures that the flux control coefficient summation theorem is satisfied.
Likewise the concentration control coefficients can also be derived, for 

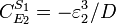

And for 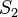

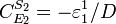

Note that the denominators remain the same as before and behave as a normalizing factor.
References
- ↑ Fell D., (1997) Understanding the Control of Metabolism, Portland Press.
- ↑ Heinrich R. and Schuster S. (1996) The Regulation of Cellular Systems, Chapman and Hall.
- ↑ Salter, M.; Knowles, R. G.; Pogson, C. I. (1994). "Metabolic control". Essays in biochemistry 28: 1–12. PMID 7925313.
- ↑ Ingalls, B. P. (2004) A Frequency Domain Approach to Sensitivity Analysis of Biochemical Systems , Journal of Physical Chemistry B, 108, 1143-1152.
- ↑ Savageau M.A (1976) Biochemical systems analysis: a study of function and design in molecular biology, Reading, MA, Addison–Wesley.
- ↑ Higgins, J. (1963). "Analysis of sequential reactions". Annals of the New York Academy of Sciences 108: 305–321. doi:10.1111/j.1749-6632.1963.tb13382.x. PMID 13954410.
- ↑ Kacser, H.; Burns, J. A. (1973). "The control of flux". Symposia of the Society for Experimental Biology 27: 65–104. PMID 4148886.
- ↑ Heinrich, R.; Rapoport, T. A. (1974). "A linear steady-state treatment of enzymatic chains. General properties, control and effector strength". European journal of biochemistry / FEBS 42 (1): 89–95. doi:10.1111/j.1432-1033.1974.tb03318.x. PMID 4830198.
- ↑ Burns, J.A., Cornish-Bowden, A., Groen, A.K., Heinrich, R., Kacser, H., Porteous, J.W., Rapoport, S.M., Rapoport, T.A., Stucki, J.W., Tager, J.M., Wanders, R.J.A. & Westerhoff, H.V. (1985) Control analysis of metabolic systems. Trends Biochem. Sci. 10, 16.