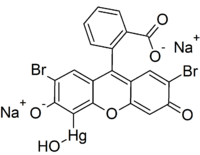
Merbromin
| Merbromin | |
|---|---|
 | |
 | |
 | |
| IUPAC name dibromohydroxymercurifluorescein | |
| Identifiers | |
| CAS number | 129-16-8 |
| ChemSpider | 10808965 |
| EC number | 204-933-6 |
| KEGG | D00861 |
| ATC code | D08 |
| Jmol-3D images | {{#if:[Na+].[Na+].[O-]C(=O)c4ccccc4C=1c3cc(Br)c([O-])c([Hg]O)c3O/C/2=C/C(=O)C(/Br)=C\C=1\2|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C20H8Br2HgNa2O6 |
| Molar mass | 750.65 g mol−1 |
| Appearance | dark green solid |
| Hazards | |
| R-phrases | R26 R27 R28 R33 R50 R53 |
| S-phrases | S13 S28 S36 S45 S60 S61 |
| Main hazards | Toxic, dangerous for the environment |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Merbromin (marketed as Mercurochrome, Merbromine, Sodium mercurescein, Asceptichrome, Supercrome, Brocasept and Cinfacromin) is a topical antiseptic used for minor cuts and scrapes. Merbromin is an organomercuric disodium salt compound and a fluorescein. It is readily available in most countries but, because of its mercury content, it is no longer sold in the United States, Germany, or France.[1][2][3]
Uses
Merbromin's best-known use is as a topical antiseptic. When applied on a wound, it stains the skin bright red. In the United States, its use has been superseded by other agents (e.g., povidone iodine, benzalkonium chloride, chloroxylenol). It is still an important antiseptic, particularly in developing nations, due to its “unbelievably low cost”.[4]
Merbromin is also used as a biological dye to mark tissue margins and as a metal dye in industrial dye penetrant inspection to detect metal fractures.
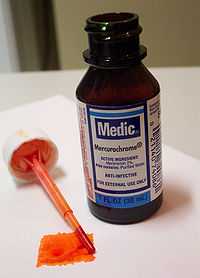
Mercurochrome
Mercurochrome is a trade name of merbromin. The name is also commonly used for over-the-counter antiseptic solutions consisting of merbromin (typically at 2% concentration) dissolved in either ethyl alcohol (tincture) or water (aqueous).
Its antiseptic qualities were discovered by Johns Hopkins Hospital physician Hugh H. Young in 1918.[5] The chemical soon became popular among parents and physicians for everyday antiseptic uses, and it was commonly used for minor injuries in the schoolyard.
The United States Food and Drug Administration (FDA) removed it from the “generally recognized as safe” and into the “untested” classification to effectively halt its distribution in the United States on October 19, 1998 over fears of potential mercury poisoning.[1] Sales were halted in Germany in 2003,[2] and in France in 2006.[3] It is readily available in most other countries.[citation needed]
Within the United States, products such as Humco Mercuroclear play on the brand recognition history of Mercurochrome but substitute other ingredients with similar properties (Mercuroclear: "Aqueous solution of benzalkonium chloride and lidocaine hydrochloride").[6]
See also
- Thiomersal, also known as Thimerosal or Merthiolate
References
- ↑ 1.0 1.1 "Quantitative and Qualitative Analysis of Mercury Compounds in the List". Federal Food, Drug, and Cosmetic Act (FD&C Act). U.S. Food and Drug Administration. 2009-04-30.
- ↑ 2.0 2.1 de:Merbromin
- ↑ 3.0 3.1 fr:Merbromine
- ↑ Mohite, P. N.; Bhatnagar, A. M. (2009). "Mercurochrome 1% as an Antiseptic for Burns: Economical - but is it Efficacious and Safe?". The Internet Journal of Surgery 21 (2). ISSN 1528-8242. "Apart from these qualities, still the most important factor for which mercurochrome has remained the favorite of the physicians in the developing countries is its attractive price. The compound is being sold at unbelievably low cost ... the reasons being the low manufacturing cost, longer shelf life, use in diluted form and importantly less propaganda about its medical use."
- ↑ Wilner, I. (2006). The Man Time Forgot: A Tale of Genius, Betrayal, and the Creation of Time Magazine. Harper Collins. p. 230. ISBN 0-06-050549-4.
- ↑ "Mercuroclear MSDS" (pdf). Humco.
| |||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||