Maltose
| Maltose | |
|---|---|
 α-Maltose | |
 β-Maltose | |
| IUPAC name 2-(hydroxymethyl)-6-[4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyox ane-3,4,5-triol | |
| Other names 4-O-α-D-Glucopyranosyl-D-glucose | |
| Identifiers | |
| CAS number | 69-79-4 |
| PubChem | 6255 |
| ChemSpider | 388329 α-maltose |
| UNII | 66Y63L379N |
| EC-number | 200-716-5 |
| ChEBI | CHEBI:17306 |
| ChEMBL | CHEMBL1234209 |
| Jmol-3D images | {{#if:O([C@H]1[C@H](O)[C@@H](O)C(O)O[C@@H]1CO)[C@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)CO|Image 1 |
| |
| |
| Properties[1] | |
| Molecular formula | C12H22O11 |
| Molar mass | 342.30 g mol−1 |
| Appearance | White powder or crystals |
| Density | 1.54 g/cm3 |
| Melting point | 160–165 °C (anhydrous) 102–103 °C (monohydrate) |
| Solubility in water | 1.080 g/mL (20 °C) |
| Chiral rotation [α]D | +140.7° (H2O, c = 10) |
| Hazards | |
| MSDS | External MSDS |
| EU Index | not listed |
| Related compounds | |
| Related | Sucrose Lactose Trehalose Cellobiose |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Maltose (/ˈmɔːltoʊs/[2] or /ˈmɔːltoʊz/[3]), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond, formed from a condensation reaction. The isomer isomaltose has two glucose molecules linked through an α(1→6) bond. Maltose is the second member of an important biochemical series of glucose chains. Maltose is the disaccharide produced when amylase breaks down starch. It is found in germinating seeds such as barley as they break down their starch stores to use for food. It is also produced when glucose is caramelized.[4]
History
Maltose was discovered by Ireland's Cornelius O’Sullivan in 1872[5] and its name comes from malt, from Old English mealt, of Germanic origin, and the suffix –ose, a suffix forming names of sugars and other carbohydrates.[6]
Structure and nomenclature
Maltose is a biomolecule that belongs to the group of carbohydrates within the division into three groups, which are divided into essential elements: carbohydrates, lipids and proteins. Carbohydrates are composed by O, H, C, and are defined as polyhydroxyaldehydes or polyhydroxyketones.
It is generally divided into monosaccharides, oligosaccharides and polysaccharides depending on the number of residues. Maltose is a disaccharide formed by the union of two glucose units (monosaccharide). The two are classified as hexoses because each one is composed of six carbons. The two glucoses which compose maltose are cyclized in piran form and are joined by an O-glycosidic bond through one of the first glucose carbon and fourth carbon of the second glucose, indicated as (1 → 4). The link is characterized as α due to the -OH position of the anomeric carbon in the opposite plane of CH2OH radical (the carbon is the number 6).
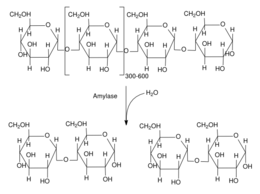
Due to O-glycosidic link, maltose is a disaccharide that can reduce Fehling's reagent. Furthermore, maltose can be obtained by hydrolysis of glycogen or starch, polymers of linked maltoses in position α (1 → 4) and branching in position α (1 → 6). These are very abundant and form a large number of branches. Amylase enzymes produce maltose and limit dextrin. These can be further degraded by maltase enzyme to hydrolyze maltoses as glucoses and they are ready to be degrade and obtain energy in form of ATP.
An isomer of maltose is isomaltose. This is similar to maltose but instead of bonds in position α (1 → 4), the linkage is formed in position α (1 → 6), therefore, glycogen branching is defined by isomaltose. Maltose is also the reducing character.
Properties
- Maltose has the ability to reduce the Fehling’s solution, due to its free aldehyde. The aldehyde group is oxidized giving a positive result, which means that the maltose is a reducing sugar.
- Maltose in aqueous solution exhibit mutarotation, due to its anomeric carbon which can form α and β isomers. In aquose solution, it is shown a balanced way between α and β forms.
- It has a sweet taste.
- Lu and Sharkey – in 2006 – said that maltose was the main carbon form exported from chloroplasts at night.[7]
Maltose intolerance
Homopolysaccharides of glucose are broken down by maltase and isomaltase to produce maltose. The lack of the Sucrase-Isomaltase enzyme, one of the four integral glycoproteins, causes sucrose and maltose intolerance and is called Congenital Sucrase-Isomaltase Deficiency. This congenital disorder is most prominent in infancy but can develop later in life. It is caused by a recessive autosomal mutation (mostly SI and SII). Nonetheless, depending on the type of mutation there are certain degrees of intolerance. According to this degree, physicians may decide the amount of starch the patient may have to remain healthy. This intolerance begins when infants begin to eat nutrients that contain starch, mainly containing maltose. One can notice that a patient suffers from this pathology when they have “stomach cramps, bloating, excess gas production, nausea, vomiting and diarrhea. Upper respiratory tract and viral infections are common. These digestive problems can lead to lower than normal weight gain and growth. Possible associations are kidney stones and copper malabsorption.”[8] Further studies have shown that certain patients with different degrees of mutation can actually improve throughout the years, reaching a maximum improvement at ages 3 to 6 months.
Sources and absorption

Maltose is a component of malt, a substance which is obtained in the process of allowing grain to soften in water and germinate. It is found in beverages, beer, cereal, pasta, potatoes and in many processed products which have been sweetened.
The production of maltose is based on the hydrolysis of starch, compound by glucose units α(1→4) and α(1→6) linked, meaning that the carbon number 1 is linked by a glycosidic bond to the carbon number 4 or 6 of the other glucose. The 1→4 linked starch part is called amylose and it is a linear polymer while the 1→4 and 1→6 linked part is the amylopectin, a branched chain polymer, being 1→6 the branching point linkages.
This kind of hydrolysis is catalyzed by enzymes called amylases,[9] classified into α-amylases and β-amylases. In the human pancreas the digestion of starch is catalyzed by an α-amylase, as the salivary enzyme. This enzyme can work with amylose and/or amylopectin, but only the interior α(1→4) glucose linkages of the amylopectin molecules as the α-amylase cannot hydrolyze the α(1→6) branching points. The impossibility of hydrolyzing these linkages affects the amylase action: it is weaker near them.[10]
The β-amylases are found in plants, sweet potatoes, soybeans, barley and wheat and are also in bacteria. Theses amylases produce β-maltose and β-limit dextrins, as a result of the enzyme’s impossibility of hydrolyzing α(1→6) branch linkages.[11]
In humans, maltose is broken down by the enzyme maltase so that there are two glucose molecules from which the glucose metabolism obtains energy.
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-367. ISBN 0-8493-0462-8..
- ↑ Dictionary Reference: maltose
- ↑ Cambridge dictionary: maltose
- ↑ Sugisawa, Hirqshi; Edo, Hiroshi (1966). "The Thermal Degradation of Sugars I. Thermal Polymerization of Glucose". Journal of Food Science 31 (4): 561. doi:10.1111/j.1365-2621.1966.tb01905.x.
- ↑
- ↑ Oxford dictionaries: http://oxforddictionaries.com
- ↑ "The importance of maltose in transitory starch breakdown". Retrieved 2014-01-12.
- ↑ Food intolerances: http://www.foodintolerances.com.au/food-intolerances-sucrose-maltose.aspx
- ↑ Biochemistry. Regiland Garrett, Charles M. Grisham.
- ↑ Textbook of Biochemistry and Human Biology. G. P. Talwar, L. M. Srivastava.
- ↑ Starch: Chemistry and Technology. James N. BeMiller, Roy L. Whistler.
External links
- Maltose, Elmhurst College Virtual Chembook
- Sucrose and maltose (starch) intolerance, Food Intolerance Diagnostics
- Congenital Sucrase-Isomaltase Deficiency (CSID
Parent Support Group]
- About CSID, sucraid.net
| |||||||||||||||||||||||||||||||||||||