Malonic acid
| Malonic acid | |
|---|---|
 | |
 | |
| IUPAC name propanedioic acid | |
| Other names methanedicarboxylic acid | |
| Identifiers | |
| CAS number | 141-82-2 |
| PubChem | 867 |
| ChemSpider | 844 |
| DrugBank | DB02175 |
| ChEBI | CHEBI:30794 |
| ChEMBL | CHEMBL7942 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C3H4O4 |
| Molar mass | 104.06 g mol−1 |
| Density | 1.619 g/cm3 |
| Melting point | 135–136 °C |
| Boiling point | decomposes |
| Solubility in water | Miscible |
| Acidity (pKa) | pKa1 = 2.83[1] pKa2 = 5.69[1] |
| Related compounds | |
| Other anions | malonate |
| Related carboxylic acids | acetic acid oxalic acid propionic acid tartronic acid acrylic acid butyric acid succinic acid fumaric acid |
| Related compounds | propanone propionaldehyde propanedial dimethyl malonate |
| Hazards | |
| MSDS | External MSDS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
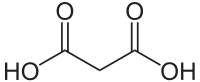
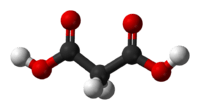
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.
Biochemistry
The calcium salt of malonic acid occurs in high concentrations in beetroot. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain.
Preparation
A classical preparation of malonic acid starts from chloroacetic acid:[2]
Sodium carbonate generates the sodium salt, which is then reacted with sodium cyanide to provide the cyano acetic acid salt via a nucleophilic substitution. The nitrile group can be hydrolyzed with sodium hydroxide to sodium malonate, and acidification affords malonic acid.
Organic reactions
In a well-known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a −CH2COOH synthon in the malonic ester synthesis.
References
- ↑ 1.0 1.1 pKa Data Compiled by R. Williams (pdf; 77 kB)
- ↑ Nathan Weiner, "Malonic acid", Org. Synth.; Coll. Vol. 2: 376
External links
- pH-Spectrum of Disodium Malonate
- pH-Spectrum of Malonate-copper-complex
- Calculator: Water and solute activities in aqueous malonic acid
