Magnetoception

Magnetoception (or magnetoreception as it was first referred to in 1972[1]) is a sense which allows an organism to detect a magnetic field to perceive direction, altitude or location. This sense has been proposed to explain animal navigation in vertebrates and insects, and as a method for animals to develop regional maps. For the purpose of navigation, magnetoception deals with the detection of the Earth's magnetic field.
Magnetoception has been observed in invertebrates such as bacteria, fruit flies and honeybees. It has also been demonstrated in vertebrates including birds, turtles, lobsters, sharks and stingrays. Magnetoception in humans is controversial.
Proposed mechanisms
An unequivocal demonstration of the use of magnetic fields for orientation within an organism has been in a class of bacteria known as magnetotactic bacteria. These bacteria demonstrate a behavioural phenomenon known as magnetotaxis, in which the bacteria orients itself and migrates in the direction along the Earth's magnetic field lines. The bacteria contain magnetosomes, which are particles of magnetite or iron sulfide enclosed within the bacteria cells.[2] Each bacterium cell essentially acts as a magnetic dipole. They form in chains where the moments of each magnetosome align in parallel, giving the bacteria its permanent-magnet characteristics. These chains are formed symmetrically to preserve the crystalline structure of the cells.[3] These bacteria are said to have permanent magnetic sensitivity.
For animals the mechanism for magnetoception is unknown, but there exist two main hypotheses to explain the phenomenon.[4] According to one model, cryptochrome, when exposed to blue light, becomes activated to form a pair of two radicals (molecules with a single unpaired electron) where the spins of the two unpaired electrons are correlated. The surrounding magnetic field affects the kind of this correlation (parallel or anti-parallel), and this in turn affects the length of time cryptochrome stays in its activated state. Activation of cryptochrome may affect the light-sensitivity of retinal neurons, with the overall result that the bird can "see" the magnetic field.[5] The Earth's magnetic field is only 0.5 Gauss and so it is difficult to conceive of a mechanism by which such a field could lead to any chemical changes other than those affecting the weak magnetic fields between radical pairs.[6] Cryptochromes are therefore thought to be essential for the light-dependent ability of the fruit fly Drosophila melanogaster to sense magnetic fields.[7]
The second proposed model for magnetoreception relies on Fe3O4, also referred to as iron (II, III) oxide or magnetite, a natural oxide with strong magnetism. Iron (II, III) oxide remains permanently magnetized when its length is larger than 50 nm and becomes magnetized when exposed to a magnetic field if its length is less than 50 nm.[8] In both of these situations the Earth's magnetic field leads to a transducible signal via a physical effect on this magnetically sensitive oxide.
Another less general type of magnetic sensing mechanism in animals that has been thoroughly described is the inductive sensing methods used by sharks, stingrays and chimaeras (cartilaginous fish). These species possess a unique electroreceptive organ known as ampullae of Lorenzini which can detect a slight variation in electric potential. These organs are made up of mucus-filled canals that connect from the skin's pores to small sacs within the animal's flesh that are also filled with mucus. The ampullae of Lorenzini are capable of detecting DC currents and have been proposed to be used in the sensing of the weak electric fields of prey and predators. These organs could also possibly sense magnetic fields, by means of Faraday's law: as a conductor moves through a magnetic field an electric potential is generated. In this case the conductor is the animal moving through a magnetic field, and the potential induced depends on the time varying rate of flux through the conductor according to
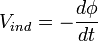 .
.These organs detect very small fluctuations in the potential difference between the pore and the base of the electroreceptor sack. An increase in potential results in a decrease in the rate of nerve activity, and a decrease in potential results in an increase in the rate of nerve activity. This is analogous to the behavior of a current carrying conductor; with a fixed channel resistance, an increase in potential would decrease the amount of current detected, and vice versa. These receptors are located along the mouth and nose of sharks and stingrays.
In invertebrates

The mollusc Tochuina tetraquetra (formerly Tritonia diomedea or Tritonia gigantea) has been studied for clues as to the neural mechanism behind magnetoreception in a species. Some of the earliest work with Tochuina showed that prior to a full moon Tochuina would orient their bodies between magnetic north and east.[9] A Y-maze was established with a right turn equal to geomagnetic south and a left turn equal to geomagnetic east. Within this geomagnetic field 80% of Tochuina made a turn to the left or magnetic east. However, when a reversed magnetic field was applied that rotated magnetic north 180° there was no significant preference for either turn, which now corresponded with magnetic north and magnetic west. These results, though interesting, do not conclusively establish that Tochuina uses magnetic fields in magnetoreception. These experiments do not include a control for the activation of the Rubens’ coil in the reversed magnetic field experiments. Therefore, it is possible that heat or noise generated by the coil was responsible for the loss of choice preference. Further work with Tochuina was unable to identify any neurons that showed rapid changes in firing as a result of magnetic fields.[10][11] However, pedal 5 neurons, two bisymmetric neurons located within the Tochuina pedal ganglion, exhibited gradual changes in firing over time following 30 minutes of magnetic stimulation provided by a Rubens’ coil. Further studies showed that pedal 7 neurons in the pedal ganglion were inhibited when exposed to magnetic fields over the course of 30 minutes. The function of both pedal 5 neurons and pedal 7 neurons is currently unknown.
.jpg)
Drosophila melanogaster is another invertebrate which may be able to orientate to magnetic fields. Experimental techniques such as gene knockouts have allowed a closer examination of possible magnetoreception in these fruit flies. Various Drosophila strains have been trained to respond to magnetic fields.[7] In a choice test flies were loaded into an apparatus with two arms that were surrounded by electric coils. Current was run through each of the coils, but only one would a 5 Gauss magnetic field at a time. The flies in this T-maze were tested on their native ability to recognize the presence of the magnetic field in an arm and on their response following training where the magnetic field was paired with a sucrose reward. Many of the strains of flies showed a learned preference for the magnetic field following training. However, when the only cryptochrome found in Drosophila, type 1 Cry, is altered, either through a missense mutation or replacement of the Cry gene, the flies exhibit a loss of magnetosensitivity. Furthermore, when light is filtered to only allow wavelengths greater than 420 nm through, Drosophila loses its trained response to magnetic fields. This response to filtered light is likely linked to the action spectrum of fly-cryptochrome which has a range from 350 nm – 400 nm and plateaus from 430-450 nm.[12] Although researchers had believed that a tryptophan triad in cryptochrome was responsible for the free radicals on which magnetic fields could act, recent work with Drosophila has shown that tryptophan might not be behind cryptochrome dependent magnetoreception. Alteration of the tryptophan protein does not result in the loss of magnetosensitivity of a fly expressing either type 1 Cry or the cryptochrome found in vertebrates, type 2 Cry.[13] Therefore it remains unclear exactly how cryptochrome mediates magnetoreception. These experiments used a 5 gauss magnetic field, 10 times the strength of the Earth's magnetic field). Drosophila has not yet been shown to be able to respond to the Earth’s weaker magnetic field.
In homing pigeons
Homing pigeons have been known to use magnetic fields as part of their complex navigation system.[14] Work by William Keeton showed that homing pigeons that were time shifted were unable to orient themselves correctly on a clear sunny day. This was considered a result of the fact that homing pigeons who used the sun for navigation would have to compensate for its movement throughout the day and a time shifted pigeon would be incapable of doing such compensation properly. However, if time shifted pigeons were released on overcast day they navigated correctly. This led to the hypothesis that under particular conditions homing pigeons rely on magnetic fields to orient themselves. Further experiments with magnets attached to the backs of homing pigeons demonstrated that disruption of the bird’s ability to sense the Earth's magnetic field leads to a loss of proper orientation behavior under overcast conditions.[15] There have been two mechanisms implicated in homing pigeon magnetoreception : the visually mediated free-radical pair mechanism and a magnetite based directional compass or inclination compass.[16] More recent behavioral tests have shown that pigeons are able to detect magnetic anomalies of 186 microtesla (1.86 Gauss).[17]
In a choice test birds were trained to jump onto a platform on one end of a tunnel if there was no magnetic field present and to jump onto a platform on the other end of the tunnel if a magnetic field was present. In this test, birds were rewarded with a food prize and punished with a time penalty. Homing pigeons were able to make the correct choice 55%-65% of the time which is higher than what would be expected if the pigeons were simply guessing. The ability of pigeons to detect a magnetic field is impaired by application of ligocaine, an anesthetic, to the olfactory mucosa. Furthermore, sectioning the trigeminal nerve leads to an inability to detect a magnetic field, while sectioning of the olfactory nerve has no effect on the magnetic sense of homing pigeons. These results suggest that magnetite located in the beak of pigeons may be responsible for magnetoreception via trigeminal mediation. However, it has not been shown that the magnetite located in the beak of pigeons is capable of responding to a magnetic field with the Earth’s strength.[18] Therefore the receptor responsible for magnetosensitivity in homing pigeons has not been cemented.
Aside from the sensory receptor for magnetic reception in homing pigeons there has been work on neural regions that are possibly involved in the processing of magnetic information within the brain. Areas of the brain that have shown increases in activity in response to magnetic fields with a strength of 50 or 150 microtesla are the posterior vestibular nuclei, dorsal thalamus, hippocampus, and visual hyperpallium.[19]
As previously mentioned, pigeons provided some of the first evidence for the use of magnetoreception in navigation. As a result, they have been an organism of focus in magnetoreception studies. The precise mechanism used by pigeons has not been established and so it is unclear yet whether pigeons rely solely on a cryptochrome-mediated receptor or on beak-magnetite.
In domestic hens
Domestic hens have iron mineral deposits in the dendrites in the upper beak and are capable of magnetoreception.[20][21] Because hens use directional information from the magnetic field of the earth to orient in relatively small areas, this raises the possibility that beak-trimming (removal of part of the beak to reduce injurious pecking frequently performed on egg-laying hens) impairs the ability of hens to orient in extensive systems, or to move in and out of buildings in free-range systems.[22]
In mammals

Work with mice, mole rats, and bats has shown that some mammals may be capable of magnetoception. When woodmice are removed from their home area and deprived of visual and olfactory cues they seem to orient themselves correctly towards their homes until an inverted magnetic field is applied to their cage.[23] When the same mice are allowed access to visual cues however they demonstrate the ability to orient themselves towards home, despite the presence of inverted magnetic fields. This seems to suggest that woodmice use magnetic fields to orient themselves when displaced if there are no other cues available. However studies such as this have been criticized because of the difficulty of completely removing sensory cues and the fact that while some of these studies are done the magnetic field is artificially changed before the test as opposed to during the test.[24] Due to the timing of the magnetic fields activation the results of these experiments do not conclusively show that woodmice respond to magnetic fields when deprived of other cues.
Work with the Zambian mole rat, a subterranean mammal, has led to reports that they use magnetic fields as a polarity compass to aid in the orientation of their nests.[24] In contrast to work with woodmice, Zambian mole rats do not exhibit different orientation behavior when a visual cue such as the sun is present, a result that has been suggested is due to their subterranean lifestyle. Further investigation of mole rat magnetoreception lead to the finding that exposure to magnetic fields leads to an increase in neural activity within the superior colliculus as measured by immediate early gene expression.[25] The activity level of neurons within two levels of the superior colliculus, the outer sublayer of the intermediate gray layer and the deep gray layer, were elevated in a non-specific manner when exposed to various magnetic fields. However, within the inner sublayer of the intermediate gray layer (InGi) there were two or three clusters of responsive cells. The more time the mole rats were exposed to a magnetic field the greater the immediate early gene expression within the InGi. However, if Zambian mole rats were placed in a field with a shielded magnetic field only a few scattered cells were active. Therefore it has been proposed that in mammals the superior colliculus is an important neural structure in the processing of magnetic information.
Bats also seem to utilize magnetic fields in orienting themselves. While bats have been known to utilize echolocation to undergo navigation over short-distances it is unclear what they use to navigate over longer distances.[26] When Eptesicus fuscus are taken from their home roosts and exposed to magnetic fields 90 degrees clockwise or counterclockwise of magnetic north, they are disoriented and set off for their homes in the wrong direction. Therefore, it seems that Eptesicus fuscus is capable of magnetosensation. However, the exact use of magnetic fields in Eptesicus fuscus is unclear as the magnetic field could be being used either as a map, a compass, or a compass calibrator. Recent work with another bat species, Myotis myotis, supports the idea that bats use magnetic fields as a compass calibrator, and their primary compass is the sun.[27]
There is evidence for magnetoreception in large mammals. Resting and grazing cattle as well as roe deer (Capreolus capreolus) and red deer (Cervus elaphus) tend to align their body axes in the geomagnetic North-South (N-S) direction.[28] Because wind, sunshine, and slope could be excluded as common ubiquitous factors in this study, alignment toward the vector of the magnetic field provided the most likely explanation for the observed behaviour. However, because of the descriptive nature of this study, alternative explanations (e.g., the sun compass) could not be excluded. In a follow-up study, researchers analyzed body orientations of ruminants in localities where the geomagnetic field is disturbed by high-voltage power lines to determine how local variation in magnetic fields may affect orientation behaviour. This was done by using satellite and aerial images of herds of cattle and field observations of grazing roe deer. Body orientation of both species was random on pastures under or near power lines. Moreover, cattle exposed to various magnetic fields directly beneath or in the vicinity of power lines trending in various magnetic directions exhibited distinct patterns of alignment. The disturbing effect of the power lines on body alignment diminished with the distance from the conductors.[29] In 2011 a group of Czech researchers, however, reported their failed attempt to replicate the finding using different Google Earth images.[30]
In humans
Magnetic bones have been found in the human nose, specifically the sphenoidal/ethmoid sinuses.[31] Beginning in the late 1970s, the group of Robin Baker at the University of Manchester began to conduct experiments that purported to exhibit magnetoception in humans: people were disoriented and then asked about certain directions; their answers were more accurate if there was no magnet attached to their head.[32] Other scientists have maintained they could not reproduce these results.[32][33] Some other evidence for human magnetoception was put forward in a 2007 study: low-frequency magnetic fields can produce an evoked response in the brains of human subjects.[34]
Magnetoception in humans has also been achieved by magnetic implants [citation needed] and by non-permanently attached artificial sensory "organs".[35] However, these exercises do little to demonstrate that humans are innately capable of magnetoreception.
Additionally, a magnetosensitive protein, cryptochrome-2, has been found in the human eye.[36] Given the lack of knowledge as to how cryptochrome mediates magnetosensitivity in Drosophila, it is unclear whether the cryptochrome found in humans functions in the same way and can be used for magnetoception.
Issues
The largest issue affecting verification of an animal magnetic sense is that despite more than 40 years of work on magnetoception there has yet to be an identification of a sensory receptor.[16] Given that the entire receptor system could likely fit in a one-millimeter cube and have a magnetic content of less than one ppm, it is difficult to discern the parts of the brain where this information is processed.[37] In various organisms a cryptochrome mediated receptor has been implicated in magnetoception. At the same time a magnetite system has been found to be relevant to magnetosensation in birds. Furthermore, it is possible that both of these mechanisms play a role in magnetic field detection in animals. This dual mechanism theory in birds raises the questions, if such a mechanism is actually responsible for magnetoception, to what degree is each method responsible for stimulus transduction, and how do they lead to a tranducible signal given a magnetic field with the Earth’s strength.[6]
The precise purpose of magnetoception in animal navigation is unclear. Some animals appear to use their magnetic sense as a map, compass, or compass calibrator. The compass method allows animals not only to find north, but also to maintain a constant heading in a particular direction. Although the ability to sense direction is important in migratory navigation, many animals also have the ability to sense small fluctuations in earth’s magnetic field to compute coordinate maps with a resolution of a few kilometers or better.[38] For example birds such as the homing pigeon are believed to use the magnetite in their beaks to detect magnetic signposts and thus, the magnetic sense they gain from this pathway is a possible map.[6] Yet, it has also been suggested that homing pigeons and other birds use the visually mediated cryptochrome receptor as a compass.[6]
The purpose of magnetoception in birds and other animals may be varied, but has proved difficult to study, and evidence remains weak. Numerous studies use magnetic fields larger than the Earth’s field. Studies such as of Tritonia have used electrophysiological recordings from only one or two neurons, and many others have been solely correlational.[citation needed]
See also
References
- ↑ M. LINDAUER & H. MARTIN in S. R. Galler et al. Animal Orientation & Navigation 559/1
- ↑ Blakemore, R. (1975). "Magnetotactic Bacteria". Science 190 (4212): 377–379. Bibcode:1975Sci...190..377B. doi:10.1126/science.170679. PMID 170679.
- ↑ The Magneto-Lab. "Biochemistry and molecular biology of magnetosome formation in Magnetospirillum gryphiswaldense." Available: http://magnum.mpi-bremen.de/magneto/research/index.html.
- ↑ Wolfgang Wiltschko, Roswitha Wiltschko (August 2008). "Magnetic orientation and magnetoreception in birds and other animals". Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 191 (8): 675–93. doi:10.1007/s00359-005-0627-7. PMID 15886990.
- ↑ Cryptochrome and Magnetic Sensing, Theoretical and Computational Biophysics Group at the University of Illinois at Urbana-Champaign. Accessed 13 February 2009
- ↑ 6.0 6.1 6.2 6.3 Rodgers, C. T.; Hore, P. J. (2009). "Chemical magnetoreception in birds: the radical pair mechanism". Proceedings of the National Academy of Sciences of the United States of America 106 (2): 353–60. Bibcode:2009PNAS..106..353R. doi:10.1073/pnas.0711968106. PMC 2626707. PMID 19129499.
- ↑ 7.0 7.1 Gegear, Robert J.; Amy Casselman, Scott Waddell, Steven M. Reppert (August 2008). "Cryptochrome mediates light-dependent magnetosensitivity in Drosophila". Nature 454 (7207): 1014–8. Bibcode:2008Natur.454.1014G. doi:10.1038/nature07183. PMC 2559964. PMID 18641630.
- ↑ Cadiou, Hervé; McNaughton, Peter A (2010). "Avian magnetite-based magnetoreception: a physiologist's perspective". Journal of the Royal Society Interface (The Royal Society) 7 (Suppl 2): S193–205. doi:10.1098/rsif.2009.0423.focus.
- ↑ Lohmann, K. J.; Willows, A. O. D. (1987). "Lunar-Modulated Geomagnetic Orientation by a Marine Mollusk". Science 235 (4786): 331–334. Bibcode:1987Sci...235..331L. doi:10.1126/science.3798115. PMID 3798115.
- ↑ Lohmann, K. J.; Willows; Pinter, R. B. (1991). "An identifiable molluscan neuron responds to changes in earth-strength magnetic fields". The Journal of experimental biology 161: 1–24.
- ↑ Wang, J. H. (2004). "Identifiable neurons inhibited by Earth-strength magnetic stimuli in the mollusc Tritonia diomedea". Journal of Experimental Biology 207 (6): 1043–1049. doi:10.1242/jeb.00864.
- ↑ VanVickle-Chavez, S.J.; Van Gelder, R. N. (2007). "Action Spectrum of Drosophila Cryptochrome *". Journal of Biological Chemistry 282 (14): 10561–10566. doi:10.1074/jbc.M609314200. PMID 17284451.
- ↑ Gegear, R. J.; Foley, L. E.; Casselman, A.; Reppert, S. M. (2010). "Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism". Nature 463 (7282): 804–7. Bibcode:2010Natur.463..804G. doi:10.1038/nature08719. PMC 2820607. PMID 20098414.
- ↑ Walcott, C. (1996). "Pigeon homing: observations, experiments and confusions". The Journal of experimental biology 199 (Pt 1): 21–7. PMID 9317262.
- ↑ Keeton, W. T. (1971). "Magnets interfere with pigeon homing". Proceedings of the National Academy of Sciences of the United States of America 68 (1): 102–6. Bibcode:1971PNAS...68..102K. doi:10.1073/pnas.68.1.102. PMC 391171. PMID 5276278.
- ↑ 16.0 16.1 Gould, J. L. (1984). "Magnetic field sensitivity in animals". Annual review of physiology 46: 585–98. doi:10.1146/annurev.ph.46.030184.003101. PMID 6370118.
- ↑ Mora, C. V.; Davison, M.; Wild, J. M.; Walker, M. M. (2004). "Magnetoreception and its trigeminal mediation in the homing pigeon". Nature 432. doi:10.1038/nature03039.1.
- ↑ Mouritsen, H.; Ritz, T. (2005). "Magnetoreception and its use in bird navigation". Current Opinion in Neurobiology 15 (4): 406–14. doi:10.1016/j.conb.2005.06.003. PMID 16006116.
- ↑ Wu, L.-Q.; Dickman, J. D. (2011). "Magnetoreception in an avian brain in part mediated by inner ear lagena". Current biology 21 (5): 418–23. doi:10.1016/j.cub.2011.01.058. PMC 3062271. PMID 21353559.
- ↑ Falkenberg, G., Fleissner, G., Schuchardt, K., Kuehbacher, M., Thalau, P., Mouritsen, H., Heyers, D., Wellenreuther, G. and Fleissner. G., (2010). Avian magnetoreception: Elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE 5:e9231
- ↑ Wiltschko, W., Freire, R., Munro, U., Ritz, T., Rogers, L.J., Thalau,P., and Wiltschko. R., (2007). The magnetic compass of domestic chicken, Gallus gallus. Journal Experimental Biology, 210:2300–2310
- ↑ Freire, R., Eastwood, M.A. and Joyce, M., (2011). Minor beak trimming in chickens leads to loss of mechanoreception and magnetoreception. Journal of Animal Science, 89:1201–1206
- ↑ Mather, J.G.; Baker, R. R. (1981). "Magnetic sense of direction in woodmice for route-based navigation". Nature 291 (5811): 152–155. Bibcode:1981Natur.291..152M. doi:10.1038/291152a0.
- ↑ 24.0 24.1 Marhold, S.; Wiltschko, W.; Burda, H. (1997). "A Magnetic Polarity Compass for Direction Finding in a Subterranean Mammal". Naturwissenschaften 84 (9): 421–423. Bibcode:1997NW.....84..421M. doi:10.1007/s001140050422.
- ↑ Nemec, P.; Altmann, J.; Marhold, S.; Burda, H.; Oelschlager, H. H. (2001). "Neuroanatomy of magnetoreception: the superior colliculus involved in magnetic orientation in a mammal". Science 294 (5541): 366–8. Bibcode:2001Sci...294..366N. doi:10.1126/science.1063351. PMID 11598299.
- ↑ Holland, R.A.; Thorup, K.; Vonhof, M.J.; Cochran, W.W.; Wikelski, M. (2006). "Bat orientation using Earth's magnetic field". Nature 444 (7120): 702. Bibcode:2006Natur.444..702H. doi:10.1038/444702a.
- ↑ Wiltschko, R.; Wiltschko, W. (2006). "Magnetoreception". BioEssays 28 (2): 157–68. doi:10.1002/bies.20363. PMID 16435299.
- ↑ Begall, S., Cerveny, J., Neef, J., Vojtech, O. and Burda, H., (2008). Magnetic alignment in grazing and resting cattle and deer. Proc. Natl. Acad. Sci. U.S.A., 105: 13451–13455
- ↑ Burda, H., Begalla, S., Červený, J., Neefa, J. and Němecd, P., (2009). Extremely low-frequency electromagnetic fields disrupt magnetic alignment of ruminants. Proc. Nat. Acad. Sci. USA, 106: 5708–5713
- ↑ Hert, J; Jelinek, L; Pekarek, L; Pavlicek, A (2011). "No alignment of cattle along geomagnetic field lines found". Journal of Comparative Physiology 197 (6): 677–682.
- ↑ Baker, R R; J G Mather, J H Kennaugh (1983-01-06). "Magnetic bones in human sinuses". Nature 301 (5895): 79–80. Bibcode:1983Natur.301...78R. doi:10.1038/301078a0. PMID 6823284.
- ↑ 32.0 32.1 Baker, R. Robin (1989). Human navigation and magnetoreception. Manchester University Press. ISBN 0-7190-2627-X.
- ↑ R. Wiltschko, W. Wiltschko (June 1995). Magnetic orientation in animals. Springer. p. 73. ISBN 3-540-59257-1.
- ↑ Carrubba, S; C Frilot, A L Chesson, A A Marino (2007-01-05). "Evidence of a nonlinear human magnetic sense". Neuroscience 144 (1): 356–67. doi:10.1016/j.neuroscience.2006.08.068. PMID 17069982.
- ↑ The feelSpace Project
- ↑ "Human eye protein senses Earth's magnetism". Science & Environment (BBC News). 21 June 2011. Retrieved 2011-06-21.
- ↑ Kirschvink, J.L. (1997). "Magnetoreception: homing in on vertebrates". Nature 390 (6658).
- ↑ Gould, J.L. (2008). "Animal navigation: the evolution of magnetic orientation". Current Biology 18 (11).
External links
- Cryptochrome and magnetic sensing, Theoretical and Biophysical Computations Group, University of Illinois at Urbana–Champaign
- Schiff H (1991). "Modulation of spike frequencies by varying the ambient magnetic field and magnetite candidates in bees (Apis mellifera)". Comp Biochem Physiol a Comp Physiol 100 (4): 975–85. doi:10.1016/0300-9629(91)90325-7. PMID 1685393.
- Johnsen S, Lohmann KJ (September 2005). "The physics and neurobiology of magnetoreception". Nature Reviews Neuroscience 6 (9): 703–12. doi:10.1038/nrn1745. PMID 16100517.