Magnesium sulfite
From Wikipedia, the free encyclopedia
| Magnesium sulfite | |
|---|---|
| |
 |
| IUPAC name Magnesium sulfite | |
| Other names Magnesium sulphite | |
| Identifiers | |
| CAS number | 7757-88-2 |
| PubChem | 3014583 |
| ChemSpider | 2282946 |
| EC number | 231-825-6 |
| Properties | |
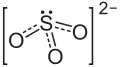
| Molecular formula | MgSO 3 (anhydrous); MgSO 3·6H 2O |
| Molar mass | 104.368200 g/mol (anhydrous) 212.4599 g/mol (heptahydrate) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula MgSO
3. Its most common hydrated form has 6 water molecules making it a hexahydrate, MgSO
3·6H
2O. When heated above 40 °C (104 °F), it is dehydrated to magnesium sulfite trihydrate, or MgSO
3·3H
2O.[1] The anhydrous form is hygroscopic, meaning that it readily absorbs water from the air.
References
- ↑ Nývlt, J., "Solubilities of Magnesium Sulfite," Journal of Thermal Analysis and Calorimetry, Volume 66, Number 2 / November, 2001
See also
- Magnesium sulfate, a.k.a. Epsom salt
| |||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.