Maghemite
| Maghemite | |
|---|---|
 Maghemite from Gancedo, Chaco Province, Argentina | |
| General | |
| Category | Oxide minerals |
| Formula (repeating unit) | γ-Fe2O3 |
| Strunz classification | 04.BB.15 |
| Crystal symmetry |
Isometric tetartoidal H-M symbol: (2 3) Space group: P 213 |
| Unit cell | a = 8.33 Å; Z = 8 or a = 8.35 Å c = 24.99 Å; Z = 32 for tetragonal supercell |
| Identification | |
| Color | Brown, bluish black; brown to yellow in transmitted light; white to bluish gray in reflected light. |
| Crystal habit | Rarely as minute octahedral crystals, or acicular overgrowths; commonly as coatings on or replacements of magnetite; massive. |
| Crystal system | Cubic with a tetragonal supercell |
| Cleavage | None |
| Fracture | Subconchoidal |
| Mohs scale hardness | 5 |
| Luster | Dull |
| Streak | Brown |
| Diaphaneity | Opaque, transparent in thin fragments |
| Specific gravity | 4.860 (calculated) |
| Optical properties | Isotropic |
| Other characteristics | Strongly magnetic |
| References | [1][2][3] |
Maghemite (Fe2O3, γ-Fe2O3) is a member of the family of iron oxides. It has the same structure as magnetite, that is, it is spinel ferrite and is also ferrimagnetic.
Maghemite can be considered as an Fe(II)-deficient magnetite with formula
![({\mathrm {Fe}}_{8}^{{\mathrm {III}}})_{A}[{\mathrm {Fe}}_{{40/3}}^{{{\mathrm {III}}}}\square _{{8/3}}]_{B}{\mathrm {O}}_{{32}}](/2014-wikipedia_en_all_02_2014/I/media/7/7/0/7/77070285986e98a0f745fe0b1e7782e9.png) [4] where
[4] where
 represents a vacancy,
represents a vacancy, 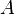 indicates tetrahedral positioning and
indicates tetrahedral positioning and  octahedral.
octahedral.
Occurrence
Maghemite forms by weathering or low-temperature oxidation of spinels containing iron(II) such as magnetite or titanian magnetite. It occurs as widespread yellow pigment in terrestrial sediments and soils. It is associated with magnetite, ilmenite, anatase, pyrite, marcasite, lepidocrocite and goethite.[1]
Maghemite was named in 1927 for an occurrence at the Iron Mountain mine, northwest of Redding, Shasta County, California.[3] The name alludes to somewhat intermediate character between MAGnetite and HEMatite. It is blue with a grey shade, white, or brown.[5] It has isometric crystals.[2] Maghemite is formed by the topotactic oxidation of magnetite.
Cation distribution
There is experimental[6] and theoretical[7] evidence that Fe(III) cations and vacancies tend to be ordered in the octahedral sites, in a way that maximizes the homogeneity of the distribution and therefore minimizes the electrostatic energy of the crystal.
Electronic structure
Maghemite is a semiconductor with a bandgap of around 2 eV,[8] although the precise value of the gap depends on the electron spin.[7]
Applications
Maghemite exhibits ferrimagnetic ordering with a high Néel temperature (~950 K), which together with its low cost and chemical stability led to its wide application as a magnetic pigment in electronic recording media since the 1940s.[9]
Maghemite nanoparticles are also used in biomedicine, because they are biocompatible and non-toxic to humans, while their magnetism allows remote manipulation with external fields.[10]
See also
- List of Minerals
References
- ↑ 1.0 1.1 Handbook of Mineralogy
- ↑ 2.0 2.1 Maghemite on Mindat
- ↑ 3.0 3.1 Maghemite on Webmineral
- ↑ R. M. Cornell and Udo Schwertmann: The iron oxides: structure, properties, reactions, occurrences, and uses, pp 32. Wiley-VCH, 2003
- ↑ Richard V. Gaines, H. Catherine W. Skinner, Eugene E. Foord, Brian Mason, and Abraham Rosenzweig: "Dana's new mineralogy", pp. 229-230. John Wiley & Sons, 1997
- ↑ C. Greaves, J. Solid State Chem. 49 325 (1983)
- ↑ 7.0 7.1 R. Grau-Crespo, A. Y. Al-Baitai, I. Saaudoune, N.H. de Leeuw, "Vacancy ordering and electronic structure of γ -Fe2O3 (maghemite): a theoretical investigation" J. Phys. Condens. Matter 22, 255401 (2010) http://iopscience.iop.org/0953-8984/22/25/255401
- ↑ M. I. Litter and M. A. Blesa Can. J. Chem. 70, 2502 (1992)
- ↑ R. Dronskowski, "The little maghemite story: A classic functional material" Adv. Funct. Mater. 11, 27 (2001) http://onlinelibrary.wiley.com/doi/10.1002/chin.200125209/abstract
- ↑ Q. A. Pankhurst, J. Connolly, S. K. Jones and J. Dobson, "Applications of magnetic nanoparticles in biomedicine" J. Phys. D: Appl. Phys. 36, R167 (2003)