Longifolene
| (+)-Longifolene | |
|---|---|
 | |
| IUPAC name (1R,2S,7S,9S)- 3,3,7-trimethyl- 8-methylenetricyclo- [5.4.0.02,9]undecane | |
| Identifiers | |
| CAS number | 475-20-7 |
| ChemSpider | 1406720 |
| Jmol-3D images | {{#if:C1(=C)[C@]2(CCCC([C@@H]3[C@@H]1CC[C@@H]23)(C)C)C|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C15H24 |
| Molar mass | 204.36 g/mol |
| Density | 0.928 g/cm3 |
| Boiling point | 254 °C (706 mm Hg) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Longifolene is the common (or trivial) chemical name of a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated,[1] Pinus longifolia (obsolete name for Pinus roxburghii Sarg.)[2]
Chemically, longifolene is a tricyclic sesquiterpene. This molecule is chiral, and the enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.
Longifolene is used in organic synthesis for the preparation of dilongifolylborane,[3] a chiral hydroborating agent.
Longifolene is also one of two most abundant aroma constituents of lapsang souchong tea, because the tea is smoked over pine fires.[4]
Total syntheses
Due to the compact tricyclic structure and lack of functional groups, Longifolene is an attractive target for research groups highlighting new synthetic methodologies. Notable syntheses are by Corey,[5][6] McMurray,[7] Johnson,[8] Oppolzer,[9] and Schultz.[10]
 |
| Longifolene total synthesis by Corey.svg |
|---|
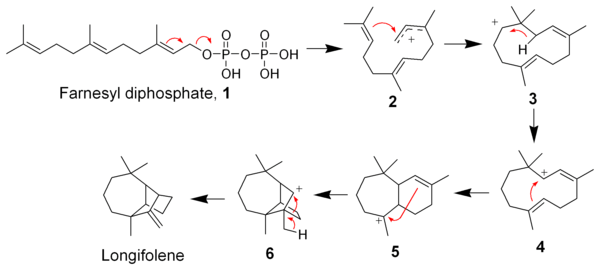
The Johnson biosynthesis has since been validated as feasible using modern quantum mechanical computational methods. The subsequent cationic cascade mechanism has been shown to go through a non-classical cation intermediate.[11]
Biosynthesis
The biosynthesis of longifolene begins with farnesyl diphosphate (1) (also called farnesyl pyrophosphate) by means of a cationic polycyclization cascade. Loss of the pyrophosphate group and cyclization by the distal alkene gives intermediate 3, which by means of a 1,3-hydride shift gives intermediate 4. After two additional cyclizations, intermediate 6 produces longifolene by a 1,2-alkyl migration.
Use
The borane derivative dilongifolylborane is used in organic synthesis as a chiral hydroborating agent.[12]
External links
References
- ↑ Naffa, P.; Ourisson, G. Bulletin de la Société chimique de France, 1954, 1410.
- ↑ Simonsen, J. L. J. Chem. Soc. 1920, 117, 570.
- ↑ Jadhav, P. K.; Brown, H. C. J. Org. Chem. 1981, 46, 2988.
- ↑ Shan-Shan Yao; Wen-Fei Guo; YI Lu; Yuan-Xun Jiang, "Flavor Characteristics of Lapsang Souchong and Smoked Lapsang Souchong,a Special Chinese Black Tea with Pine Smoking Process", Journal of Agricultural and Food Chemistry, Vol. 53, No.22, (2005)
- ↑ Corey, E. J. et al. J. Am. Chem. Soc. 1961, 83, 1251.
- ↑ Corey, E. J. et al. J. Am. Chem. Soc. 1964, 86, 478.
- ↑ McMurray, J. E.; Isser, S. J. J. Am. Chem. Soc. 1972, 94, 7132.
- ↑ Volkermann, R. A.; Andrews, G. C.; Johnson, W. S. J. Am. Chem. Soc. 1975, 97, 4777-4779.
- ↑ Oppolzer, W.; Godel, T. J. Am. Chem. Soc. 1978, 100, 2583.
- ↑ Schultz, A. G. et al. J. Org. Chem. 1985, 50, 915.
- ↑ Ho, Gregory J. Org. Chem. 2005, 70, 5139 -5143.
- ↑ Dev, Sukh (1981). "Aspects of longifolene chemistry. An example of another facet of natural products chemistry". Accounts of Chemical Research 14 (3): 82–88.