Lithium amide
| Lithium amide | ||
|---|---|---|
| IUPAC name Lithium amide | ||
| Other names Lithamide | ||
| Identifiers | ||
| CAS number | 7782-89-0 | |
| PubChem | 24532 | |
| ChemSpider | 22939 | |
| Jmol-3D images | {{#if:[Li+].[NH2-]|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | LiNH2 | |
| Molar mass | 22.96 g/mol | |
| Appearance | white solid | |
| Density | 1.178 g/cm3 | |
| Melting point | 390 °C | |
| Boiling point | 430 °C | |
| Solubility in water | decomposes | |
| Hazards | ||
| NFPA 704 |
 1
3
2
| |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Lithium amide is an inorganic compound with the chemical formula Li+NH2-, i.e. it is composed of a lithium cation, and the conjugate base of ammonia. It is a white solid with a tetragonal crystal structure.
Lithium amides
The anionic conjugate bases of amines are known as amides. Thus lithium amide may also refer to lithium salts of amines e.g. Li+NR2-. An example of a lithium amide is lithium diisopropylamide (LDA), which is quite commonly used.
Lithium amide can be made by adding lithium metal to liquid ammonia:
- 2Li + 2NH3 → 2LiNH2 + H2
Lithium amides in general can be similarly formed, substituting ammonia with the appropriate amine:
- 2Li + 2R2NH → 2LiNR2 + H2
Lithium amides are very reactive compounds and can act as strong bases. Unless the nitrogen atom is hindered, as in the case of LDA, they can also act as nucleophiles.
Examples
The lithium salt of 2,2,6,6-tetramethylpiperidine has been crystallised as a tetramer:
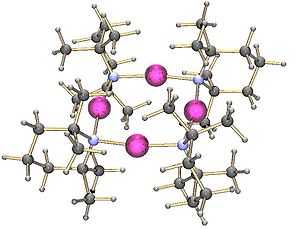
On the other hand, the lithium derivative of di-(1-phenylethyl)amine crystallises as a trimer:

It is also possible to make mixed oligomers of metal alkoxides and amides.[1] These are related to the superbases which are mixtures of metal alkoxides and alkyls. The cyclic oligomers form when the nitrogen of the amide forms a sigma bond to a lithium while the nitrogen lone pair binds to another metal centre.
Other organolithium compounds (such as BuLi) are generally considered to exist in and function via high-order, aggregated species.
See also
- Sodium amide
- Butyllithium
- Lithium hexamethyldisilazide
References
- ↑ K.W. Henderson, D.S. Walther and P.G. Williard (1995). "Identification of a Unimetal Complex of Bases by 6Li NMR Spectroscopy and Single-Crystal Analysis". Journal of the American Chemical Society 117 (33): 8680–8681. doi:10.1021/ja00138a030.
- Merck Index, 11th Edition, 5398.
External links
| |||||||||||