Lipoxin
| Lipoxin B4 | |
|---|---|
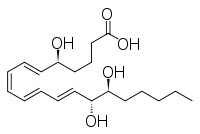 | |
| IUPAC name 5S,14R,15S-Trihydroxy-6E,8Z,10E,12E -eicosatetraenoic acid | |
| Other names LXB4 | |
| Identifiers | |
| CAS number | 92950-25-9 |
| PubChem | 5280915 |
| ChemSpider | 4444430 |
| ChEBI | CHEBI:6499 |
| Jmol-3D images | {{#if:O=C(O)CCC[C@H](O)/C=C/C=C\C=C\C=C\[C@@H](O)[C@@H](O)CCCCC|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C20H32O5 |
| Molar mass | 352.46508 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |

Lipoxins are members of the family of bioactive products generated from Arachidonic Acid (AA). They have a number of immunomodulatory and anti-inflammatory actions. Lipoxins are short lived endogenously produced nonclassic eicosanoids whose appearance in inflammation signals the resolution of inflammation. They are abbreviated as LX, an acronym for lipoxygenase (LO) interaction products. At present two lipoxins have been identified; lipoxin A4 (LXA4) and lipoxin B4 (LXB4).
History
Lipoxins were first described by Serhan, Hamberg and Samuelsson in 1984.[1] They reported that the lipoxins stimulated superoxide anion (O2−) generation and degranulation at submicromolar concentrations—as potent as LTB4.
Biosynthesis
Lipoxins are derived enzymatically from arachidonic acid, an ω-6 fatty acid. Transcellular biosynthetic mechanisms play a key role in their production. They are formed by platelets but they alone cannot synthesize them. Platelets depend upon neutrophils for LTA4, which is converted to LXA4 and LXB4 by the action of platelet 12-lipoxygenase. LTC4, LTD4, and LTE4 are also synthesized in platelets from LTA4. An analogous class, the resolvins, are derived from EPA and DHA, ω-3 fatty acids.[1] Another analogous class, the epi-lipoxins, are formed by non-enzymatic peroxidation.
Biological activity
Lipoxins, as well as certain peptides, are high affinity ligands for the lipoxin A4 receptor (ALX), which was first identified based on sequence homology as the formyl peptide receptor like receptor (FPRL1). At least 6 homologues of this promiscuous receptor are found in mice. Lipoxin signaling through the LXA4R inhibits chemotaxis, transmigration, superoxide generation and NF-κB activation.[2] Conversely, peptide signaling through the same receptor, in vitro, has been shown to stimulate chemotaxis of polymorphonuclear cells (PMNs) and calcium mobilization.[2] The peptides that have ALXR affinity tend to be signals for leukocyte migration and subsequent phagocytosis such as acute phase proteins, bacterial peptides, HIV envelope proteins and neurotoxic peptides.
At higher concentrations, LXA4 has been shown to bind AhR, the arylhydrocarbon receptor, but only in murine cells.[3] More recently, LXA4 was demonstrated to bind Estrogen receptor alpha and to act as an estrogenic molecule in human endometrial epithelial cells in vitro and in mouse uterine tissue in vivo.[4]
Similarly to the leukotrienes, LXA4 will form the cysteinyl-lipoxins LXC4, LXD4 and LXE4.[5] At subnanomolar concentrations, LXA4 and LXB4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells.[6] The receptor for LXB4 has not been identified.
Lipoxins are high affinity antagonists to the cysteinyl leukotriene receptor type 1 (CysLT1) to which several leukotrienes (LTC4, LTD4 and LTE4) mediate their smooth muscle contraction and eosinophil chemotactic effects. The CysLT1 receptor is also the site of action for the asthma drug montelukast (Singulair).[7]
In resolution
During inflammation, cells die by apoptosis. As part of resolution, lipoxins signal macrophages to the remains of these cells (phagocytosis).[8] During the acute inflammatory process, the proinflammatory cytokines such as IFN-γ and IL-1β can induce the expression of anti-inflammatory mediators such as lipoxins and IL-4, which promote the resolution phase of inflammation.[9]
Lipoxin analogues
Stable synthetic analogues of LXs and aspirin-triggered 15-epi-LXA4s (ATLs) can mimic many of the desirable anti-inflammatory, "pro-resolution" actions of native LXs.[10]
References
- ↑ 1.0 1.1 Charles N. Serhan, Mats Hamberg, and Bengt Samuelsson (September 1, 1984). "Lipoxins: Novel Series of Biologically Active Compounds Formed from Arachidonic Acid in Human Leukocytes" (pdf). Retrieved 2006-02-02. Original description of lipoxins.
- ↑ 2.0 2.1 Chiang N., Arita M., and Serhan CN. (2005). "Anti-inflammatory circuitry: Lipoxin, aspirin-triggered lipoxins and their receptor ALX". Prostaglandins, Leukotrienes and Essential Fatty Acids 73 (3–4): 163–177. doi:10.1016/j.plefa.2005.05.003. PMID 16125378.
- ↑ Biochemistry. 1999 Jun 8;38(23):7594-600.Lipoxin A4: a new class of ligand for the Ah receptor.Schaldach CM, Riby J, Bjeldanes LF. PMID: 10360957
- ↑ Lipoxin A4 is a novel estrogen receptor modulator.Russell R, Gori I, Pellegrini C, Kumar R, Achtari C, Canny GO.FASEB J. 2011 Dec;25(12):4326-37. doi: 10.1096/fj.11-187658. PMID: 21885654
- ↑ Powell WS, Chung D, Gravel S (1995). "5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration". J. Immunol. 154 (8): 4123–32. PMID 7706749.
- ↑ Papayianni A, Serhan CN, Brady HR (1996). "Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells". J. Immunol. 156 (6): 2264–72. PMID 8690917.
- ↑ Drazen J., Israel E., and O'Byrne P. (N Engl J Med. 1999 January 21;340(3):197-206). "Treatment of Asthma with Drugs Modifying the Leukotriene Pathway". The New England Journal of Medicine 340 (3): 197–206. doi:10.1056/NEJM199901213400306. PMID 9895400.
- ↑ Mitchell S, Thomas G, Harvey K, et al. (2002). "Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo". J. Am. Soc. Nephrol. 13 (10): 2497–507. doi:10.1097/01.ASN.0000032417.73640.72. PMID 12239238.
- ↑ McMahon, Blaithin and Godson, Catherine (Am J Physiol Renal Physiol 286: F189-F201, 2004). "Lipoxins: endogenous regulators of inflammation". Retrieved 2006-02-07. Invited review article.
- ↑ McMahon B, Mitchell S, Brady HR (2001). "Lipoxins: revelations on resolution". Trends Pharmacol. Sci. 22 (8): 391–5. doi:10.1016/S0165-6147(00)01771-5. PMID 11478982.
External links
- Lipoxins at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||||||||||||||||||||||||||