Linaclotide
 | |
|---|---|
| Systematic (IUPAC) name | |
| L-Cysteinyl-L-cysteinyl-L-glutamyl-L-tyrosyl-L-cysteinyl-L-cysteinyl-L-asparaginyl-L-prolyl-L-alanyl-L-cysteinyl-L-threonylglycyl-L-cysteinyl-L-tyrosine cyclo(1-6),(2-10),(5-13)-tris(disulfide) | |
| Clinical data | |
| Trade names | Linzess |
| Licence data | US FDA:link |
| Pregnancy cat. | C (US) |
| Legal status | ℞-only (US) |
| Routes | Oral |
| Identifiers | |
| CAS number | 851199-59-2 |
| ATC code | A06AX04 |
| PubChem | CID 16158208 |
| ChemSpider | 17314504 |
| UNII | N0TXR0XR5X |
| KEGG | D09355 |
| Chemical data | |
| Formula | C59H79N15O21S6 |
| Mol. mass | 1526.74 g/mol |
| SMILES
| |
| |
| | |
Linaclotide (marketed under the trade name Linzess and Constella) is an experimental peptide agonist of guanylate cyclase 2C that is undergoing clinical trials for use in treating abdominal pain in patients with irritable bowel syndrome (IBS) accompanied by constipation. The drug also has promising outlooks for the treatment of gastroparesis, ulcerative colitis, chronic intestinal pseudo-obstruction (CIPO), and inertia coli as well.[1] The drug was developed by Ironwood Pharmaceuticals, based in Cambridge, Massachusetts.
Linaclotide was approved by the FDA on August 30, 2012 for the treatment of chronic idiopathic constipation and to treat irritable bowel syndrome with constipation (IBS-C) in adults.[2] It became available in US pharmacies on December 17, 2012.[3] That same month, it was forecast by market research firm Decision Resources to achieve blockbuster status by 2021.[4]
The National Institutes of Health (NIH) estimates that as many as 20% of Americans may experience signs of irritable bowel syndrome, with approximately one-third of those affected experiencing constipation often accompanied by abdominal pain, affecting as many as 10 million Americans. Laxatives can assist with constipation but don't treat pain, while use of opiates to treat pain can aggravate constipation. While low-cost laxatives and pain killers would likely be tried first, linaclotide targets both associated conditions in a once-daily pill and could be used if standard treatments are unsuccessful in treating symptoms, though it would likely cost as much as several dollars per day.[5]
Clinical trials
In Phase I trials reported in January 2009 in The American Journal of Gastroenterology, researchers found that 42 patients with chronic constipation who participated in the randomized, double-blind, placebo-controlled study experienced relief and that the medication was well tolerated.[6] In results of a first round of Phase III clinical trials announced in September 2010, Ironwood studied approximately 800 patients over 12 weeks who were given linaclotide or a placebo in a randomized double-blind trial. 34% of those receiving linaclotide experienced relief of pain and constipation, compared to 21% of patients who had taken the placebo. 50% of those receiving linaclotide saw a significant reduction in pain, versus 37% with the placebo, with pain reduction starting in the first week on the medication. 6% of patients left the study after experiencing diarrhea, the most commonly reported side effect.[5]
Distribution and licensing
Under a partnership agreement announced in 2007 between Forest Laboratories and Microbia (as Ironwood was then known), Forest would pay $70 million in licensing fees towards the development of linaclotide, with profits shared between the two companies.[7] Distribution rights in the United States will be shared with Forest Laboratories, with Almirall distributing linaclotide in Europe and Astellas Pharma in Asia.[5]
Chemistry
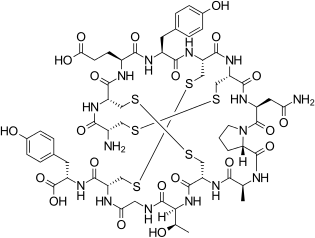
Linaclotide is a peptide consisting of 14 amino acids. The sequence is
H–Cys1–Cys2–Glu3–Tyr4–Cys5–Cys6–Asn7–Pro8–Ala9–Cys10–Thr11–Gly12–Cys13–Tyr14–OH
There are three disulfide bonds: Between Cys1 and Cys6, between Cys2 and Cys10, and between Cys5 and Cys13.[8]
Other drugs against irritable bowel syndrome
As of 2012, lubiprostone (marketed by Sucampo and Takeda under the trade name Amitiza) is the only other medication besides linaclotide with Food and Drug Administration approval to treat IBS with constipation.[9] The Novartis drug tegaserod (marketed as Zelnorm) had received FDA approval in 2002 for treatment of IBS, but was taken off the market after studies found an increased risk of heart attack and stroke associated with the drug.
References
- ↑ Tadataka Yamada, ed. (2011). Textbook of Gastroenterology. John Wiley & Sons. ISBN 9781444359411.
- ↑ "FDA approves Linzess to treat certain cases of irritable bowel syndrome and constipation". 30 Aug 2012.
- ↑ "Ironwood and Forest Announce U.S. Availability of LINZESS". 17 Dec 2012.
- ↑ "Constella/Linzess Will Achieve Blockbuster Sales of $1.2 Billion in 2021 in the Irritable Bowel Syndrome Drug Market". 19 Dec 2012.
- ↑ 5.0 5.1 5.2 Pollack, Andrew. "Drug for Irritable Bowel Achieves Goals in Trial", The New York Times, September 13, 2010. Accessed September 14, 2010.
- ↑ Jeffrey M Johnston , Caroline B Kurtz , Douglas A Drossman , Anthony J Lembo , Brenda I Jeglinski , James E MacDougall , Stephen M Antonelli & Mark G Currie . "Pilot Study on the Effect of Linaclotide in Patients With Chronic Constipation", The American Journal of Gastroenterology 104, 125–132 (1 January 2009) | doi:10.1038/ajg.2008.59. Accessed September 15, 2010.
- ↑ Staff. "Daily International Pharma Alert", FDANews, September 17, 2007, Vol. 4 No. 182. Accessed September 15, 2010.
- ↑ Albericio, F; Giraud, M; Gongora, M; Paradis, M; Tulla-Puche, J; Werbitzky, O. Solid-Phase Synthesis of the Cys-rich Peptide Linaclotide.
- ↑ "FDA approves Linzess, new drug for irritable bowel, constipation". Los Angeles Times. 31 August 2012.
External link
Linzess information Drugs com Retrieved 10-23-2013.
| |||||||||||||||||||||||||||||||
