Lignan
The lignans are a group of chemical compounds found in plants. Lignans are one of the major classes of phytoestrogens, which are estrogen-like chemicals and also act as antioxidants. The other classes of phytoestrogens are isoflavones and coumestans. Plant lignans are polyphenolic substances derived from phenylalanine via dimerization of substituted cinnamic alcohols (see cinnamic acid), known as monolignols, to a dibenzylbutane skeleton 2. This reaction is catalysed by oxidative enzymes and is often controlled by dirigent proteins.
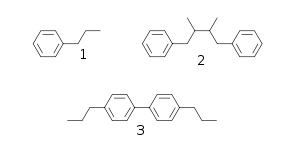
Many natural products, known as phenylpropanoids, are built up of C6C3 units (a propylbenzene skeleton 1) derived from cinnamyl units just as terpene chemistry builds on isoprene units. Structure 3 is a neolignan, a structure formed by joining the two propylbenzene residues at other than the β-carbon atom of the propyl side chain.[1]
Some examples of lignans are pinoresinol, podophyllotoxin, and steganacin.
When part of the human diet, some lignans are metabolized to form mammalian lignans known as enterodiol (1) and enterolactone (2) by intestinal bacteria. Lignans that can be metabolized to form mammalian lignans are pinoresinol, lariciresinol, secoisolariciresinol, matairesinol, hydroxymatairesinol, syringaresinol and sesamin.

Food sources
Flax seed and sesame seed contain higher levels of lignans than most other foods. The principal lignan precursor found in flaxseed is secoisolariciresinol diglucoside. Other sources of lignans include cereals (rye, wheat, oat and barley - rye being the richest source), soybeans, cruciferous vegetables such as broccoli and cabbage, and some fruits, particularly apricots and strawberries.[2]
Secoisolariciresinol and matairesinol were the first plant lignans identified in foods. Pinoresinol and lariciresinol are more recently identified plant lignans that contribute substantially to the total dietary lignan intakes. Typically, Lariciresinol and pinoresinol contribute about 75% to the total lignan intake whereas secoisolariciresinol and matairesinol contribute only about 25%.[2] This distribution may change as the contributions of syringaresinol and hydroxymatairesinol have not properly been quantified in foods.
Sources of lignans:[3]
| Source | Amount per 100 g |
|---|---|
| Flaxseed | 300,000 µg (0.3 g) |
| Sesame seed | 29,000 µg (29 mg) |
| Brassica vegetables | 185 - 2321 µg |
| Grains | 7 - 764 µg |
| Red wine | 91 µg |
A recent study[4] shows the complexity of mammalian lignan precursors in the diet. In the table below are a few examples of the 22 analyzed species and the 24 lignans identified in this study.
Mammalian lignan precursors as aglycones (µg / 100 g). Major compound(s) in bold.
| Foodstuff | Pinoresinol | Syringaresinol | Sesamin | Lariciresinol | Secoisolariciresinol | Matairesinol | Hydroxymatairesinol |
|---|---|---|---|---|---|---|---|
| Flaxseed | 871 | 48 | not detected | 1780 | 165759 | 529 | 35 |
| Sesame seed | 47136 | 205 | 62724 | 13060 | 240 | 1137 | 7209 |
| Rye bran | 1547 | 3540 | not detected | 1503 | 462 | 729 | 1017 |
| Wheat bran | 138 | 882 | not detected | 672 | 868 | 410 | 2787 |
| Oat bran | 567 | 297 | not detected | 766 | 90 | 440 | 712 |
| Barley bran | 71 | 140 | not detected | 133 | 42 | 42 | 541 |
Potential pharmacology
Lignans are also good antioxidants scavenging free radicals that may play a role in some diseases like cardiovascular disease .[5] Neolignans have been reported to directly bound and activate the peroxisome proliferator-activated receptor gamma (PPAR gamma)..[6]
See also
|
|
References
Footnotes
- ↑ neolignane at en.wiktionary
- ↑ 2.0 2.1 Linus Pauling Institute at Oregon State University
- ↑ Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (2005). "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". Br. J. Nutr. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- ↑ Smeds AI, et al.; Eklund, Patrik C.; Sjöholm, Rainer E.; Willför, Stefan M.; Nishibe, Sansei; Deyama, Takeshi; Holmbom, Bjarne R. (2007). "Quantification of a Broad Spectrum of Lignans in Cereals, Oilseeds, and Nuts". J. Agric. Food Chem. 55 (4): 1337–1346. doi:10.1021/jf0629134. PMID 17261017.
- ↑ Liu H. (2011). "Extraction and Isolation of compounds from Herbal medicines". In Willow JH Liu. Traditional Herbal Medicine Research Methods. John Wiley and Sons, Inc.
- ↑ Fakhrudin, N.; Ladurner, A.; Atanasov, A. G.; Heiss, E. H.; Baumgartner, L.; Markt, P.; Schuster, D.; Ellmerer, E. P. et al. (2010). "Computer-Aided Discovery, Validation, and Mechanistic Characterization of Novel Neolignan Activators of Peroxisome Proliferator-Activated Receptor ". Molecular Pharmacology 77 (4): 559–66. doi:10.1124/mol.109.062141. PMC 3523390. PMID 20064974.
General references
External links
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||
| |||||||||||||||||