Licochalcone A
From Wikipedia, the free encyclopedia
| Licochalcone A | ||
|---|---|---|
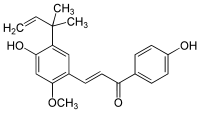 | ||
| IUPAC name (E)-3-[4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one | ||
| Other names Licochalcone a | ||
| Identifiers | ||
| CAS number | 58749-22-7 | |
| PubChem | 5318998 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C21H22O4 | |
| Molar mass | 338.39 g/mol | |
| Hazards | ||
| R-phrases | R20, R21, R22 | |
| S-phrases | S36 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Licochalcone A is a chalconoid, a type of natural phenols. It can be isolated from root of Glycyrrhiza glabra[1] or Glycyrrhiza inflata.[2] It shows antimalarial properties in vitro.[3]
References
- ↑ Fu, Y.; Hsieh, T. C.; Guo, J.; Kunicki, J.; Lee, M. Y. W. T.; Darzynkiewicz, Z.; Wu, J. M. (2004). "Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells". Biochemical and Biophysical Research Communications 322 (1): 263–270. doi:10.1016/j.bbrc.2004.07.094. PMID 15313200.
- ↑ Friis-Møller, A.; Chen, M.; Fuursted, K.; Christensen, S. R. B. G.; Kharazmi, A. (2002). "In Vitro Antimycobacterial and Antilegionella Activity of Licochalcone a from Chinese Licorice Roots". Planta Medica 68 (5): 416–419. doi:10.1055/s-2002-32087. PMID 12058317.
- ↑ Chen, M.; Theander, T. G.; Christensen, S. B.; Hviid, L.; Zhai, L.; Kharazmi, A. (1994). "Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. Yoelii infection". Antimicrobial agents and chemotherapy 38 (7): 1470–1475. PMC 284578. PMID 7979274.
| ||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.