Lead titanate
| Lead titanate | ||
|---|---|---|
 | ||
| Other names Lead(II) titanate | ||
| Identifiers | ||
| PubChem | 16211560 | |
| Properties | ||
| Molecular formula | PbTiO3 | |
| Molar mass | 303.09 g/mol | |
| Appearance | Yellow powder | |
| Density | 7.52 g/cm3 | |
| Solubility in water | Insoluble | |
| Hazards | ||
| R-phrases | R20/22, R33, R50/53, R61, R62[1] | |
| S-phrases | S45, S53, S60, S61[1] | |
| Main hazards | Toxic (T) Dangerous for the environment (N) May damage fertility or unborn child | |
| NFPA 704 |
 0
2
0
| |
| LD50 | 12000 mg/kg (rat) | |
| Related compounds | ||
| Other anions | Lead dioxide Lead acetate | |
| Other cations | Caesium titanate Iron(II) titanate | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.
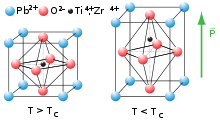
At high temperatures, lead titanate adopts a cubic perovskite structure. At 720 K,[2] the material undergoes a second order phase transition to a tetragonal perovskite structure which exhibits ferroelectricity. Lead titanate is one of the end members of the lead zirconate titanate (Pb[ZrxTi1-x]O3 0≤x≤1, PZT) system, which is technologically one of the most important ferroelectric ceramics.
Toxicity
Lead titanate is toxic, like other lead compounds. It irritates skin, mucous membranes and eyes. It may also cause harm to unborn babies and might have effects on fertility.[3]
References
- ↑ 1.0 1.1 Alfa Aesar http://www.alfa.com/en/GP100w.pgm?DSSTK=035671
- ↑ Noheda, Cereceda, Iglesias, Lifante, Gonzalo, Chen and Wang, Phys. Rev. B 51, 16388 (1995)
- ↑ http://www.alfa.com/content/msds/USA/35671.pdf
External Links
| |||||||||||