Lead-lead dating
Lead-lead dating is a method for dating geological samples, normally based on 'whole-rock' samples of material such as granite. For most dating requirements it has been superseded by uranium-lead dating (U-Pb dating), but in certain specialized situations (such as dating meteorites and the age of the Earth) it is more important than U-Pb dating.
Decay equations for common Pb-Pb dating
There are three stable "daughter" Pb isotopes that result from the radioactive decay of uranium and thorium in nature; they are 206Pb, 207Pb, and 208Pb. 204Pb is the only non-radiogenic lead isotope, therefore is not one of the daughter isotopes. These daughter isotopes are the final decay products of U and Th radioactive decay chains beginning from 238U, 235U and 232Th respectively. With the progress of time, the final decay product accumulates as the parent isotope decays at a constant rate. This shifts the ratio of radiogenic Pb versus non-radiogenic 204Pb (207Pb/204Pb or 206Pb/204Pb) in favor of radiogenic 207Pb or 206Pb. This can be expressed by the following decay equations:
where the subscripts P and I refer to present-day and initial Pb isotope ratios, λ235 and λ238 are decay constants for 235U and 238U, and t is the age.
The concept of common Pb-Pb dating (also referred to as whole rock lead isotope dating) was deduced through mathematical manipulation of the above equations.[1] It was established by dividing the first equation above by the second, under the assumption that the U/Pb system was undisturbed. This rearranged equation formed:
where the factor of 137.88 is the present-day 238U/235U ratio. As evident by the equation, initial Pb isotope ratios, as well as the age of the system are the two factors which determine the present day Pb isotope compositions. If the sample behaved as a closed system then graphing the difference between the present and initial ratios of 207Pb/204Pb versus 206Pb/204Pb should produce a straight line. The distance the point moves along this line is dependent on the U/Pb ratio, whereas the slope of the line depends on the time since Earth’s formation. This was first established by Nier et al., 1941.[2]
The formation of the Geochron
The development of the Geochron was mainly attributed to Clair Cameron Patterson’s application of Pb-Pb dating on meteorites in 1956. The Pb ratios of three stony and two iron meteorites were measured.[3] The dating of meteorites would then help Patterson in determining not only the age of these meteorites but also the age of Earth’s formation. By dating meteorites Patterson was directly dating the age of various planetesimals. Assuming the process of isotopic differentiation is identical on Earth as it is on other planets, the core of these planetesimals would be depleted of uranium and thorium, while the crust and mantle would contain higher U/Pb ratios. As planetesimals collided, various fragments were scattered and produced meteorites. Iron meteorites were identified as pieces of the core, while stony meteorites were segments of the mantle and crustal units of these various planetesimals.
-

Iron Meteorite found in Canyon Diablo
-

Meteorite Impact
Samples of iron meteorite from Canyon Diablo (Meteor Crater) Arizona were found to have the least radiogenic composition of any material in the solar system. The U/Pb ratio was so low that no radiogenic decay was detected in the isotopic composition [4]
As illustrated in figure 1, this point defines the lower (left) end of the isochron. Therefore troilite found in Canyon Diablo represents the primeval lead isotope composition of the solar system, dating back to 4.55 +- 0.07 Byr.[5] The stony meteorites however, exhibited very high 207Pb/204Pb versus 206Pb/204Pb ratios, indicating that these samples came from the crust or mantle of the planetesimal. These samples define an isochron in figure 1, whose slope gives the age of meteorites as 4.55 Byr. Patterson also analyzed terrestrial sediment collected from the ocean floor. This was representative of the Bulk Earth composition, and was plotted on the isochron. The isotope composition of this sample was found to lie on the meteorite isochron, therefore giving good evidence that earth had the same origin as the meteorites, as well as the same age, therefore solving the age of the Earth and giving rise to the name geochron.

Lead isotope isochron diagram used by C. C. Patterson to determine the age of the Earth in 1956. Animation shows progressive growth over 4550 million years (Myr) of the lead isotope ratios for two stony meteorites (Nuevo Laredo and Forest City) from initial lead isotope ratios matching those of the Canyon Diablo iron meteorite.
References
- ↑ (Nier, A.O., Thompson, R.W. and Murphy, B.F.(1941). The isotopic constitution of lead and the measurement of geological time 3. Phys. Rev. 60, 112-17. )
- ↑ ( Nier, A.O., Thompson, R.W. and Murphy, B.F.(1941). The isotopic constitution of lead and the measurement of geological time 3. Phys. Rev. 60, 112-17. )
- ↑ (Patterson, C.C. (1956). Age of meteorites and the Earth. Geochim. Cosmochim. Acta 10, 230-7)
- ↑ (Dickin, A.P, 2005.Radiogenic Isotope Geology. United Kingdom: University Press, Cambridge. p 117)
- ↑ (Dickin, A.P, 2005.Radiogenic Isotope Geology. United Kingdom: University Press, Cambridge. pp117)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

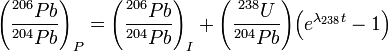
![\left[{\frac {\left({\frac {^{{207}}Pb}{^{{204}}Pb}}\right)_{{P}}-\left({\frac {^{{207}}Pb}{^{{204}}Pb}}\right)_{{I}}}{\left({\frac {^{{206}}Pb}{^{{204}}Pb}}\right)_{{P}}-\left({\frac {^{{206}}Pb}{^{{204}}Pb}}\right)_{{I}}}}\right]={\left({\frac {1}{137.88}}\right)}{\left({\frac {e^{{\lambda _{{235}}t}}-1}{e^{{\lambda _{{238}}t}}-1}}\right)}](/2014-wikipedia_en_all_02_2014/I/media/7/f/b/a/7fba5b3719f2b52bd53d4d4f8768540e.png)
