Lead(IV) acetate
| Lead(IV) acetate | ||
|---|---|---|
 | ||
| IUPAC name Lead(IV) acetate | ||
| Other names Lead tetraacetate | ||
| Identifiers | ||
| CAS number | 546-67-8 | |
| PubChem | 11025 | |
| Properties | ||
| Molecular formula | Pb(C2H3O2)4 | |
| Molar mass | 443.376 g/mol | |
| Appearance | colorless or pink crystals | |
| Odor | vinegar | |
| Density | 2.228 g/cm3 (17 °C) | |
| Melting point | 175 °C | |
| Boiling point | decomposes | |
| Solubility in water | reacts with water | |
| Solubility | reacts with ethanol soluble in chloroform, benzene, nitrobenzene, hot acetic acid, HCl, tetrachloroethane | |
| Hazards | ||
| Main hazards | Toxic | |
| NFPA 704 |
 0
3
0
| |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Lead(IV) acetate or lead tetraacetate is a chemical compound with chemical formula Pb(C2H3O2)4 and is a lead salt of acetic acid. It is commercially available often stabilized with acetic acid.
Preparation
It can be prepared by reaction of red lead with acetic acid.[1][2] The other main lead acetate is lead(II) acetate.
Reagent in organic chemistry
Lead tetraacetate is a strong oxidizing agent,[2] a source of acetyloxy groups and a general reagent for the introduction of lead into organolead compounds. Some of its many uses in organic chemistry:
- acetoxylation of benzylic, allylic and α-oxygen ether C-H bonds, for example the photochemical conversion of dioxane to 1,4-dioxene through the 2-acetoxy-1,4-dioxane intermediate [3] and the conversion of α-pinene to verbenone [4]
- an alternative reagent to bromine in Hofmann rearrangement[5]
- oxidation of hydrazones to diazo compounds for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane [6]
- aziridine formation, for example the reaction of N-aminophthalimide and stilbene [7]
- cleavage of 1,2-diols to the corresponding aldehydes or ketones often replacing ozonolysis, for instance the oxidation of di-n-butyl d-tartrate to n-butyl glyoxylate [8]
- reaction with alkenes to γ-lactones
- oxidation of alcohols carrying a δ-proton to cyclic ethers.[9]
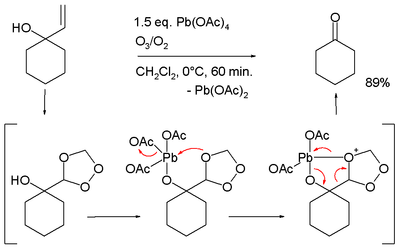
- Oxidative cleavage of certain allyl alcohols in conjunction with ozone:[10][11]
- conversion of acetophenones to phenyl acetic acids [12]
- Decarboxylation of carboxylic acids to alkyl halides in the Kochi reaction[13]
Safety
Lead(IV) acetate may be fatal if ingested, inhaled, or absorbed through skin. It causes irritation to skin, eyes, and respiratory tract. It is a neurotoxin. It affects the gum tissue, central nervous system, kidneys, blood, and reproductive system.
References
- ↑ Inorg. Synth, 1, 47 (1939).
- ↑ 2.0 2.1 J. Zýka (1966). "Analytical study of the basic properties of lead tetraacetate as oxidizing agent". Pure and Applied Chemistry 13 (4): 569–581. doi:10.1351/pac196613040569. Retrieved 19 December 2013.
- ↑ Organic Syntheses, Vol. 82, p.99 (2005) Article.
- ↑ Organic Syntheses, Coll. Vol. 9, p.745 (1998); Vol. 72, p.57 (1995) Article
- ↑ Baumgarten, Henry; Smith, Howard; and Staklis, Andris (1975). "Reactions of amines. XVIII. Oxidative rearrangement of amides with lead tetraacetate". The Journal of Organic Chemistry 40 (24): 3554–3561. doi:10.1021/jo00912a019. Retrieved 19 December 2013.
- ↑ Organic Syntheses, Coll. Vol. 6, p.161 (1988); Vol. 50, p.6 (1970) Article.
- ↑ Organic Syntheses, Coll. Vol. 6, p.56 (1988); Vol. 55, p.114 (1976) Link
- ↑ Organic Syntheses, Coll. Vol. 4, p.124 (1963); Vol. 35, p.18 (1955) Article.
- ↑ M B Smith, J March. March's Advanced Organic Chemistry (Wiley, 2001) (ISBN 0-471-58589-0)
- ↑ O3/Pb(OAc)4: a new and efficient system for the oxidative cleavage of allyl alcohols E.J. Alvarez-Manzaneda R. Chahboun , M.J. Cano, E. Cabrera Torres, E. Alvarez, R. Alvarez-Manzaneda, b, A. Haidour and J.M. Ramos López Tetrahedron Letters Volume 47, Issue 37 , 11 September 2006, Pages 6619-6622 doi:10.1016/j.tetlet.2006.07.020
- ↑ Conversion of 1-allyl-cyclohexanol to cyclohexanone, in the proposed reaction mechanism the allyl group is first converted to a trioxalane according to conventional ozonolysis which then interacts with the alkoxy lead group
- ↑ Synthesis 1981, 2, 126-127.
- ↑ A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate Jay K. Kochi J. Am. Chem. Soc.; 1965; 87(11); 2500–02. doi:10.1021/ja01089a041
| |||||||||||