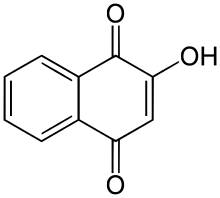
Lawsone
| Lawsone[1][2] | ||
|---|---|---|
 | ||
 | ||
| IUPAC name 2-Hydroxy-1,4-naphthoquinone | ||
| Other names Hennotannic acid | ||
| Identifiers | ||
| CAS number | 83-72-7 | |
| PubChem | 6755 | |
| UNII | TLH4A6LV1W | |
| ChEMBL | CHEMBL240963 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C10H6O3 | |
| Molar mass | 174.15 | |
| Appearance | Yellow prisms | |
| Melting point | 195-196 °C (decomposition) | |
| Solubility in water | almost insoluble [3] | |
| Hazards | ||
| R-phrases | R36 R37 R38 | |
| S-phrases | S26 S36 S37 S39 | |
| LD50 | 100 mg/kg | |
| Related compounds | ||
| Related naphthoquinones | Juglone | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant (Lawsonia inermis) as well as jewelweed (Impatiens balsamina).[4] Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5000 years. Lawsone reacts chemically with the protein known as keratin in skin and hair, in a process known as Michael addition, resulting in a strong permanent stain that lasts until the skin or hair is shed. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning and sunscreens. Chemically, lawsone is similar to juglone, which is found in walnuts.
Related compounds
The naphthoquinones lawsone methyl ether and methylene-3,3'-bilawsone are some of the active compounds in Impatiens balsamina leaves.[5]
References
- ↑ Merck Index, 12th Edition, 5406.
- ↑ MSDS at Physical & Theoretical Chemistry Laboratory, University of Oxford
- ↑ http://msds.chem.ox.ac.uk/HY/2-hydroxy-1,4-naphthaquinone.html
- ↑ Dweek, A. C. (2002). "Natural ingredients for colouring and styling". Int. J. Cosmetic Sci. 24 (5): 287–302. doi:10.1046/j.1467-2494.2002.00148.x. PMID 18498522.
- ↑ Sakunphueak A, Panichayupakaranant P (2010). "Simultaneous determination of three naphthoquinones in the leaves of Impatiens balsamina L. by reversed‐phase high‐performance liquid chromatography". Phytochem Anal 21 (5): 444–50. doi:10.1002/pca.1216. PMID 20931623.