Lactose permease
| LacY proton/sugar symporter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Pfam | PF01306 | ||||||||
| Pfam clan | CL0015 | ||||||||
| InterPro | IPR022814 | ||||||||
| PROSITE | PDOC00698 | ||||||||
| TCDB | 2.A.1 | ||||||||
| OPM superfamily | 15 | ||||||||
| OPM protein | 2cfq | ||||||||
| |||||||||
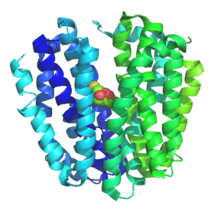
Lactose permease is a membrane protein which is a member of the major facilitator superfamily. Lactose permease can be classified as a symporter, which uses the gradient of H+ towards the cell to transport lactose in the same direction into the cell.
The protein has twelve transmembrane helices and exhibits an internal two-fold symmetry, relating the N-terminal six helices onto the C-terminal helices.
The sugar lies in a pocket in the center of the protein which is accessible from the periplasm. On binding, a large conformational change takes place which makes the sugar binding site accessible from the cytoplasm.
Mechanism: hydrogen from the outside of the cell binds to a carboxyl group on the enzyme that allows it to undergo a conformational change. This form of lactose permease can bind lactose from outside the cell. The enzyme then everts and lactose is transported inward.
The X-ray crystal structure was first solved in 2003 by J. Abramson et al. [2]
References
- ↑ Chaptal, V.; Kwon, S.; Sawaya, M. R.; Guan, L.; Kaback, H. R.; Abramson, J. (2011). "Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition". Proceedings of the National Academy of Sciences 108 (23): 9361–9366. doi:10.1073/pnas.1105687108. PMC 3111295. PMID 21593407.
- ↑ Abramson, J.; Smirnova, I.; Kasho, V.; Verner, G.; Kaback, H. R.; Iwata, S. (2003). "Structure and Mechanism of the Lactose Permease of Escherichia coli". Science 301 (5633): 610–615. doi:10.1126/science.1088196. PMID 12893935.