L-Deoxyribose
From Wikipedia, the free encyclopedia
| L-Deoxyribose | |
|---|---|
 |
 |
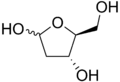
| IUPAC name (3R,4S)-3,4,5-Trihydroxypentanal | |
| Other names 2-Deoxy-L-erythro-pentose; 2-Deoxy-L-ribose; L-2-Deoxyribose | |
| Identifiers | |
| CAS number | 18546-37-7 |
| PubChem | 6994527, 9855484 hemiacetal |
| ChemSpider | 5362530 |
| Jmol-3D images | {{#if:O=CC[C@@H](O)[C@@H](O)COOC[C@@H]1OC(O)C[C@H]1O|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C5H10O4 |
| Molar mass | 134.13 g mol−1 |
| Appearance | White solid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
L-Deoxyribose is an organic compound with formula C5H10O4. It is a synthetic monosaccharide, a stereoisomer (mirror image) of the natural compound D-deoxyribose.
L-Deoxyribose can be synthesized from D-galactose.[1] It has been used in chemical research, e.g. in the synthesis of mirror-image DNA.[2]
References
- ↑ SHI Zhen-Dan, YANG Bing-Hui, and WU Yu-Lin (2002), A stereospecific synthesis of L-deoxyribose, L-ribose and L-ribosides. Tetrahedron, volume 58, issue 16, pp. 3287–3296
- ↑ Hidehito Urata, Emiko Ogura, Keiko Shinohara, Yoshiaki Ueda and Masao Akagi (1992), Synthesis and properties of mirror-image DNA. Nucleic Acids Research, volume 20 issue 13, pp. 3325-3332
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.