Krapcho decarboxylation
From Wikipedia, the free encyclopedia
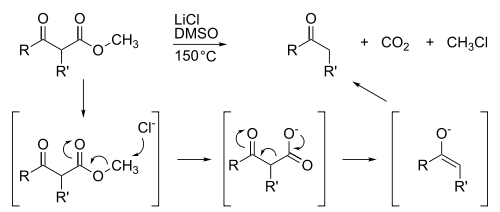
The Krapcho decarboxylation is the chemical reaction of esters with halide anions. The ester must contain an electron-withdrawing group in the beta position, such as β-ketoesters, malonic esters, α-cyanoesters, or α-sulfonylesters. It works best with methyl esters, since it is basically a SN2-reaction at carbon. It is driven by the entropy of the overall reaction, as the byproducts chloromethane and CO2 are lost as gases. The reaction is a useful synthetic procedure to hydrolyze and decarboxylate malonic esters because it only cleaves one of the ester groups. The alternative way (e.g. basic hydrolysis) destroys both of the ester groups and a subsequent reaction is usually used to regenerate the alkylated ester.[1][2][3][4]
References
- ↑ Flynn, D (1992). "Use of atom-transfer radical cyclizations as an efficient entry into a new "serotonergic" azanoradamantane". Tetrahedron Letters 33 (48): 7283. doi:10.1016/S0040-4039(00)60166-1.
- ↑ Krapcho, A. Paul; Weimaster, J. F.; Eldridge, J. M.; Jahngen, E. G. E.; Lovey, A. J.; Stephens, W. P. (1978). "Synthetic applications and mechanism studies of the decarbalkoxylations of geminal diesters and related systems effected in dimethyl sulfoxide by water and/or by water with added salts". The Journal of Organic Chemistry 43: 138. doi:10.1021/jo00395a032.
- ↑ Krapcho, A (1974). "Decarbalkoxylations of geminal diesters and β-keto esters in wet dimethyl sulfoxide. Effect of added sodium chloride on the decarbalkoxylation rates of mono- and di-substituted Malonate esters". Tetrahedron Letters 15 (13): 1091. doi:10.1016/S0040-4039(01)82414-X.
- ↑ Krapcho, A (1967). "The decarbethoxylation of geminal dicarbethoxy compounds". Tetrahedron Letters 8 (31): 215. doi:10.1016/S0040-4039(00)90519-7.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.