Kosmotropic
Solutes are defined as kosmotropic if they contribute to the stability and structure of water-water interactions. Kosmotropes cause water molecules to favorably interact, which also stabilizes intermolecular interactions in macromolecules such as proteins.
Ionic Kosmotropes
Ionic kosmotropes tend to be small or have high charge density. Some ionic kosmotropes are sulfate, phosphate, magnesium(2+), lithium(1+), zinc (2+) and aluminium (+3). Large ions or ions with low charge density (such as bromide, iodide, potassium(1+), caesium(1+)) instead act as chaotropes.
A scale can be established if one refers to the Hofmeister series or looks up the free energy of hydration (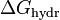 ) of the salts. The more negative
) of the salts. The more negative 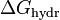 , the more kosmotropic the salt.
, the more kosmotropic the salt.
Applications
Ammonium sulfate is the traditional kosmotropic salt for the salting out of protein from an aqueous solution. Kosmotropes are used to prevent protein aggregation in pharmaceutical preparation and at various stage of protein extraction and purification. They act by stabilizing native intramolecular protein interactions, thus out-competing the intermolecular interactions that lead to aggregation.
Nonionic Kosmotropes
Nonionic kosmotropes have no net charge but are very soluble and become very hydrated. Carbohydrates such as trehalose and glucose as well as proline and tert-butanol are kosmotropes.
See also
- Chaotropic agent and Guanidinium chloride
- Protein precipitation, on aluminium sulfate "salting out"
External links
- Kosmotropes and Chaotropes (from Water Structure and Behavior)
- Polson, C; Sarkar, P; Incledon, B; Raguvaran, V; Grant, R (2003). "Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography-tandem mass spectrometry". Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 785 (2): 263–275. doi:10.1016/S1570-0232(02)00914-5. PMID 12554139.