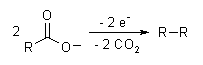Kolbe electrolysis
From Wikipedia, the free encyclopedia
"Kolbe reaction" redirects here. For the carboxylation of phenols, see Kolbe–Schmitt reaction.
The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe.[1][2] The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions) The overall general reaction is:
If a mixture of two different carboxylates are used, all combinations of them are generally seen as the organic product structures:
- R1COO− + R2COO− → R1−R1 + R1−R2 + R2−R2
The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate, then two such intermediates combine to form a covalent bond..[3] As an example, electrolysis of acetic acid yields ethane and carbon dioxide:
- CH3COOH → CH3COO− → CH3COO· → CH3· + CO2
- 2CH3· → CH3CH3
Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid:[4]
See also
References
- ↑ Kolbe, Hermann (1848). "Zersetzung der Valeriansäure durch den elektrischen Strom". Annalen der Chemie und Pharmacie 64 (3): 339–341. doi:10.1002/jlac.18480640346.
- ↑ Kolbe, Hermann (1849). "Untersuchungen über die Elektrolyse organischer Verbindungen". Annalen der Chemie und Pharmacie 69 (3): 257–372. doi:10.1002/jlac.18490690302.
- ↑ Vijh, A. K.; Conway, B. E. (1967). "Electrode Kinetic Aspects of the Kolbe Reaction". Chem Rev 67 (6): 623–664. doi:10.1021/cr60250a003.
- ↑ Sharkey, W. H.; Langkammerer, C. M. (1973), "2,7-Dimethyl-2,7-dinitrooctane", Org. Synth.; Coll. Vol. 5: 445
External links
- "Kolbe Electrolysis". Organic Chemistry Portal. Retrieved 2007-10-22.
| |||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.