Kolbe–Schmitt reaction
The Kolbe–Schmitt reaction or Kolbe process (named after Hermann Kolbe and Rudolf Schmitt) is a carboxylation chemical reaction that proceeds by heating sodium phenolate (the sodium salt of phenol) with carbon dioxide under pressure (100 atm, 125 °C), then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid (the precursor to aspirin).[1][2][3]

By using the potassium salt 4-hydroxybenzoic acid is accessible, an important precursor for the versatile paraben class of biocides used e.g. in personal care products.
Reaction mechanism
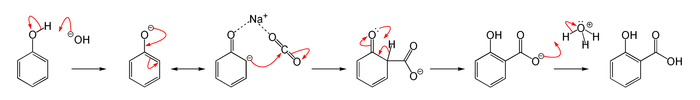
The Kolbe–Schmitt reaction proceeds via the nucleophile addition of a phenoxide, classically sodium phenoxide (NaOC6H5), to carbon dioxide to give the salicylate. The final step is reaction of the salicylate with acid to form the desired salicylic acid.
References
- ↑ Hermann Kolbe (1860). "Ueber Synthese der Salicylsäure". Annalen der Chemie und Pharmacie 113 (1): 125–27. doi:10.1002/jlac.18601130120.
- ↑ R. Schmitt (1885). "Beitrag zur Kenntniss der Kolbe'schen Salicylsäure Synthese". Journal für Praktische Chemie 31 (1): 397–411. doi:10.1002/prac.18850310130.
- ↑ A. S. Lindsey and H. Jeskey (1957). "The Kolbe-Schmitt Reaction". Chem. Rev. 57 (4): 583–620. doi:10.1021/cr50016a001. (Review)