Jasmone
| Jasmone | |
|---|---|
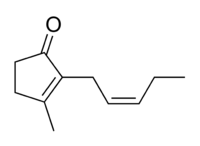 | |
| IUPAC name 3-methyl-2-[(2Z)-pent-2-en-1-yl]cyclopent-2-en-1-one | |
| Other names cis-jasmone | |
| Identifiers | |
| CAS number | 488-10-8 |
| PubChem | 1549018 |
| ChemSpider | 1266012 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C11H16O |
| Molar mass | 164.246 g/mol |
| Appearance | colorless to pale yellow liquid |
| Density | 0.94 g/mL, liquid |
| Melting point | 203-205 C |
| Boiling point | 146 °C at 27 mm Hg |
| Solubility in water | in water |
| Related compounds | |
| Related compounds | jasmonate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Jasmone is a natural organic compound extracted from the volatile portion of the oil from jasmine flowers. It is a colorless to pale yellow liquid that has the odor of jasmine. Jasmone can exist in two isomeric forms with differing geometry around the pentenyl double bond, cis-jasmone and trans-jasmone. The natural extract contains only the cis form, while synthetic material is often a mixture containing both forms, with the cis form predominating. Both forms have similar odors and chemical properties.
Jasmone is produced within plants by the metabolism of jasmonate, from linolenic acid by the octadecanoid pathway. It can act as either an attractant or a repellent for various insects. Commercially jasmone is used primarily in perfumes and cosmetics.
References
- Thomas Koch, Katja Bandemer, Wilhelm Boland (1997). "Biosynthesis of cis-Jasmone: a pathway for the inactivation and the disposal of the plant stress hormone jasmonic acid to the gas phase?". Helvetica Chimica Acta 80 (3): 838–850. doi:10.1002/hlca.19970800318.
- L. Ruzicka, M. Pfeiffer (1933). "Über Jasminriechstoffe I. Die Konstitution des Jasmons". Helvetica Chimica Acta 16: 1208–1214. doi:10.1002/hlca.193301601153.