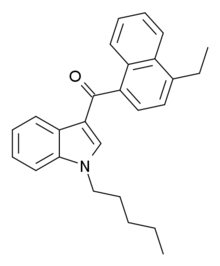
JWH-210
 | |
|---|---|
| Systematic (IUPAC) name | |
| 4-ethylnaphthalen-1-yl-(1-pentylindol-3-yl)methanone | |
| Clinical data | |
| Legal status | Illegal in Sweden,I-N(Poland)[1] |
| Identifiers | |
| CAS number | 824959-81-1 824960-02-3 (JWH-182) |
| ATC code | ? |
| PubChem | CID 45270396 |
| Chemical data | |
| Formula | C26H27NO |
| Mol. mass | 369.498 g/mol |
| SMILES
| |
| | |
JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46nM at CB1 and 0.69nM at CB2. It is one of the most potent 4-substituted naphthoyl derivatives in the naphthoylindole series, having a higher binding affinity (i.e. lower Ki) at CB1 than both its 4-methyl and 4-n-propyl homologues JWH-122 (CB1 Ki 0.69nM) and JWH-182 (CB1 Ki 0.65nM) respectively, and than the 4-methoxy compound JWH-081 (CB1 Ki 1.2nM).[2] It was discovered by and named after Dr. John W. Huffman. JWH-210 and JWH-122 were banned in Sweden on 1 October 2010 as hazardous goods harmful to health, after being identified as ingredients in "herbal" synthetic cannabis products.[3][4] The substances JWH-210, JWH-122 and JWH-203 were classified as illegal drugs by the Swedish government as of 1 September 2011.[5]
See also
References
- ↑ "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. Retrieved 12 June 2011.
- ↑ Huffman, J., et al. (2005). "Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists.". Bioorganic & Medicinal Chemistry 13 (1): 89–112. doi:10.1016/j.bmc.2004.09.050. PMID 15582455.
- ↑ Swedish Code of Statutes Regulation (2010:1086).
- ↑ Swedish Code of Statutes Regulation (2010:1086). (pdf)
- ↑ LVFS 2011:8