Indium
| Indium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 49In | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indium in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
silvery lustrous gray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | indium, In, 49 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈɪndiəm/ IN-dee-əm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | poor metal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 13, 5, p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 114.818(1) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p1 2, 8, 18, 18, 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Ferdinand Reich and Hieronymous Theodor Richter (1863) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Hieronymous Theodor Richter (1867) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.31 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 7.02 g·cm−3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 429.7485 K, 156.5985 °C, 313.8773 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2345 K, 2072 °C, 3762 °F | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 429.7445 K, ~1[1] kPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 3.281 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 231.8 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.74 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3, 2, 1 (amphoteric oxide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.78 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 558.3 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1820.7 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2704 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 167 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 142±5 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 193 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | tetragonal
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[2] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 83.7 nΩ·m | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 81.8 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 32.1 µm·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 1215 m·s−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 11 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 8.83 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-74-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of indium | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Indium is a chemical element with symbol In and atomic number 49. This rare, very soft, malleable and easily fusible poor heavy metal is chemically similar to gallium and thallium, and shows intermediate properties between these two. Indium was discovered in 1863 and named for the indigo blue line in its spectrum that was the first indication of its existence in zinc ores, as a new and unknown element. The metal was first isolated in the following year. Zinc ores continue to be the primary source of indium, where it is found in compound form. Very rarely the element can be found as grains of native (free) metal, but these are not of commercial importance.
Indium's current primary application is to form transparent electrodes from indium tin oxide (ITO) in liquid crystal displays and touchscreens, and this use largely determines its global mining production. It is widely used in thin-films to form lubricated layers (during World War II it was widely used to coat bearings in high-performance aircraft). It is also used for making particularly low melting point alloys, and is a component in some lead-free solders.
Indium is not known to be used by any organism. In a similar way to aluminium salts, indium(III) ions can be toxic to the kidney when given by injection, but oral indium compounds do not have the chronic toxicity of salts of heavy metals, probably due to poor absorption in basic conditions. Radioactive indium-111 (in very small amounts on a chemical basis) is used in nuclear medicine tests, as a radiotracer to follow the movement of labeled proteins and white blood cells in the body.
Characteristics
Physical

Indium is a very soft, silvery-white, highly ductile, relatively rare poor metal with a bright luster.[3] It is so soft (Mohs hardness 1.2) that the metal can be cut with a knife, as can sodium. It also leaves a visible line on paper.[4] Like tin, when it is bent indium emits a high-pitched "cry".[3] Like gallium, indium is able to wet glass. Like both, indium has a low melting point, 156.60 °C (313.88 °F); higher than its lighter homologue, gallium, but lower than its heavier homologue, thallium, and lower than tin. Only mercury, gallium, and most of the alkali metals have lower melting points.[5] Its boiling point is, however, moderate, being 2072 °C (3762 °F), which is higher than that of thallium, but lower than that of gallium, showing opposition to the melting points trend. Indium thus has a very large liquid range of around 2000 °C. The density of indium, 7.31 g·cm−3, is also higher than that of gallium, but lower than that of thallium. Below its critical temperature of 3.41 K, indium becomes a superconductor. At standard temperature and pressure, indium crystallizes in the tetragonal crystal system in the space group I4/mmmm (lattice parameters: a = 325 pm, c = 495 pm).[5]
Chemical
Indium is a poor metal and chemically, is the intermediate element between its group 13 neighbors gallium and thallium. An indium atom has 49 electrons, having an electronic configuration of [Kr]4d105s25p1. In its compounds, indium most commonly loses its three outermost electrons, becoming indium(III) ions, In3+, but in some cases the pair of 5s-electrons can stay within the atom, indium thus being oxidized only to indium(I), In+. This happens due to the inert pair effect, which occurs because of the stabilization of 5s-orbital due to relativistic effects, which are stronger closer to the bottom of the periodic table. In(III) is the more stable oxidation state. Thallium (indium's heavier homolog) shows an even stronger effect, making oxidation to thallium(I) more likely than to thallium(III), making +1 the more likely oxidation state,[6] while gallium (indium's lighter homolog) commonly shows only the +3 oxidation state. Thus while thallium(III) being a moderately strong oxidizing agent, indium(III) is stable and indium(I) is a powerful reducing agent.[7]
A number of standard electrode potentials, depending on the reaction under study,[8] are reported for indium:
| −0.40 | In2+ + e− | ↔ In+ |
| −0.49 | In3+ + e− | ↔ In2+ |
| −0.443 | In3+ + 2 e− | ↔ In+ |
| −0.3382 | In3+ + 3 e− | ↔ In |
| −0.14 | In+ + e− | ↔ In |
Indium does not react with water, but it is oxidized by stronger oxidizing agents, such as halogens or oxalic acid, to give indium(III) compounds. It does not react with boron, silicon or carbon, and the corresponding boride, silicide or carbide are not known. Similarly, reaction between indium and hydrogen has not been observed, but both indium(I) and indium(III) hydrides are known.[9]
Indium(III) oxide is formed at high temperatures during reaction between indium and oxygen, with blue flame. It is amphoteric, i. e. it can react with both acids and bases. Its reaction with water results in insoluble indium(III) hydroxide, which is also amphoteric, reacting with alkalies to give indates(III) and with acids to give indium(III) salts:
- In(OH)3 + 2 NaOH → 2 Na[InO2] + H2O
- In(OH)3 + 3 HCl → InCl3 + 3 H2O
The hydrolysis of sodium indate(III) gives weak indic acid, HInO2. Out of common indium(III) salts, chloride, sulfate and nitrate are soluble. In water solutions, In3+ and [InO2]- ions are hydrolyzed to give InOH2+ and HInO2 due to generally amphoteric character of indium(III) ions. Indium(III) compounds are not well-soluble, similarly to thallium(III) compounds; however, indium(III) salts of strong acids, such as chloride, sulfate and nitrate are soluble, hydrolyzing in water solutions. The In3+ ion is colorless in solution because of the absence of unpaired electrons in the d- and f-electron shells.[7]
Indium(I) compounds are not as common as indium(III) ones; only chloride, bromide, iodide, sulfide and cyclopentadienyl are well-characterized. Indium(I) sulfide is the product of reaction between indium and sulfur or indium and hydrogen sulfide, and can be received at 700—1000 °C. Indium(I) oxide black powder is received at 850 °C during reaction between indium and carbon dioxide or during decomposition of indium(III) oxide at 1200 °C. Cyclopentadienylindium(I), which was the first organoindium(I) compound reported,[10] is polymer consisting of zigzag chains of alternating indium atoms and cyclopentadienyl complexes.
Less frequently, indium shows intermediate oxidation state +2, which lies between the common ones, most notably in halides, In2X4 and [In2X6]2-.[11] Several other compounds are known to combine indium(I) and indium(III), such as InI6(InIIICl6)Cl3,[12] InI5(InIIIBr4)2(InIIIBr6),[13] InIInIIIBr4.[11] In organic synthesis it is used for indium mediated allylation.
Isotopes
Indium occurs naturally on Earth only in two primordial nuclides, indium-113 and indium-115. Indium-115 makes up 95.7% of all indium but it is radioactive, decaying to tin-115 via beta decay with half-life of 4.41×1014 years, four orders of magnitude longer than the age of the universe and nearly 50,000 times longer than that of natural thorium.[14] This situation is uncommon among stable chemical elements; only indium, tellurium, and rhenium have been shown to have most-abundant isotopes that are radioactive. The less common natural isotope of indium, indium-113, is stable.
Indium has 39 known isotopes, ranging in mass between 97 and 135. Only one of them is stable and one has half-life exceeding 1014 years; these are the only naturally occurring isotopes. The most stable artificial indium isotope is indium-111, which has half-life of approximately 2.8 days. All other isotopes have half-lives shorter than 5 hours. Indium also has 47 meta states, out of which indium-114m1 is the most stable, being more stable than the ground state of any indium isotope (except for the primordial ones).
Creation and occurrence
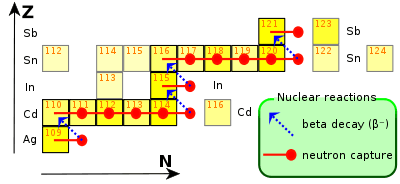
Indium is created via the long-lasting, (up to thousands of years), s-process in low-medium mass stars (which range in mass between 0.6 and 10 masses of Sun). When a silver-109 atom (the isotope of which approximately half of all silver in existence is composed), catches a neutron, it undergoes a beta decay to become cadmium-110. Capturing further neutrons, it becomes cadmium-115, which decays to indium-115 via another beta decay. This explains why the radioactive isotope predominates in abundance compared to the stable one.[15]
Indium is 61st most abundant element in the Earth's crust at approximately 49 ppb, making indium approximately as abundant as mercury.[16] Fewer than 10 indium minerals are known, such as dzhalindite (In(OH)3) and indite (FeIn2S4),[17] but none of these occurs in significant deposits.
Based on content of indium in zinc ore stocks, there is a worldwide reserve of approximately 6,000 tonnes of economically viable indium.[18] However, the Indium Corporation, the largest processor of indium, claims that, on the basis of increasing recovery yields during extraction, recovery from a wider range of base metals (including tin, copper and other polymetallic deposits) and new mining investments, the long-term supply of indium is sustainable, reliable and sufficient to meet increasing future demands.[19]
This conclusion may be reasonable considering that silver, which is one-third as abundant as indium in the Earth's crust,[20] is currently mined at approximately 18,300 tonnes per year,[21] which is 40 times greater than current indium mining rates.
History
In 1863, the German chemists Ferdinand Reich and Hieronymous Theodor Richter were testing ores from the mines around Freiberg, Saxony. They dissolved the minerals pyrite, arsenopyrite, galena and sphalerite in hydrochloric acid and distilled raw zinc chloride. As it was known that ores from that region sometimes contain thallium they searched for the green emission lines with spectroscopy. The green lines were absent but a blue line was present in the spectrum. As no element was known with a bright blue emission they concluded that a new element was present in the minerals. They named the element with the blue spectral line indium, from the indigo color seen in its spectrum.[22][23] Richter went on to isolate the metal in 1864.[24] At the World Fair 1867 an ingot of 0.5 kg (1.1 lb) was presented.[25]
In 1924, indium was found to have a valuable ability to stabilize non-ferrous metals, which was the first significant use for the element.[26] It took until 1936 for the U.S. Bureau of Mines to list indium as a commodity, and even in early the 1950s only very limited applications for indium were known, the most important of which was making light-emitting diodes and coating bearings for aircraft engines during World War II. The start of production of indium-containing semiconductors started in 1952. The development and widespread use of indium-containing nuclear control rods increased demand during the 1970s, and the use of indium tin oxide in liquid crystal displays increased and became the major application by 1992.[27][28][29][30][31]
Production

The lack of indium mineral deposits and the fact that indium is enriched in sulfidic lead, tin, copper, iron and predominately in zinc deposits, makes zinc production the main source for indium. The indium is leached from slag and dust of zinc production. Further purification is done by electrolysis. The exact process varies with the exact composition of the slag and dust.[3][25]
Indium is produced mainly from residues generated during zinc ore processing but is also found in iron, lead, and copper ores.[3] China is a leading producer of indium (390 tonnes in 2012), followed by Canada, Japan and South Korea with 70 tonnes each.[32] The Teck Cominco refinery in Trail, British Columbia, is a large single-source indium producer, with an output of 32.5 tonnes in 2005, 41.8 tonnes in 2004 and 36.1 tonnes in 2003. South American Silver Corporation's Malku Khota property in Bolivia is a large resource of indium with an indicated resource of 1,481 tonnes and inferred resource of 935 tonnes.[33] Adex Mining Inc.’s Mount Pleasant Mine in New Brunswick, Canada, holds some of the world’s total known indium resources.[34]
The amount of indium consumed is largely a function of worldwide LCD production. Worldwide production in 2007 was 475 tonnes per year from mining and a further 650 tonnes per year from recycling.[19] Demand has risen rapidly in recent years with the popularity of LCD computer monitors and television sets, which now account for 50% of indium consumption.[35] Increased manufacturing efficiency and recycling (especially in Japan) maintain a balance between demand and supply. According to the UNEP, indium's end-of-life recycling rate is less than 1%.[36] Demand increased as the metal is used in LCDs and televisions, and supply decreased when a number of Chinese mining concerns stopped extracting indium from their zinc tailings. In 2002, the price was US$94 per kilogram. The recent changes in demand and supply have resulted in high and fluctuating prices of indium, which from 2006 to 2009 ranged from US$382/kg to US$918/kg.
It has been estimated that there are fewer than 14 years left of indium supplies, based on current rates of extraction, demonstrating the need for additional recycling.[37]
Applications

The first large-scale application for indium was as a coating for bearings in high-performance aircraft engines during World War II. Afterward, production gradually increased as new uses were found in fusible alloys, solders, and electronics. In the 1950s, tiny beads of it were used for the emitters and collectors of PNP alloy junction transistors. In the middle and late 1980s, the development of indium phosphide semiconductors and indium tin oxide thin films for liquid crystal displays (LCD) aroused much interest. By 1992, the thin-film application had become the largest end use.[38][39]
Electronics
- Indium oxide (In2O3) and indium tin oxide (ITO) are used as a transparent conductive coating applied to glass substrates in the making of electroluminescent panels.[40]
- Some indium compounds such as indium antimonide, indium phosphide,[41] and indium nitride[42] are semiconductors with useful properties.
- Indium is used in the synthesis of the semiconductor copper indium gallium selenide (CIGS), which is used for the manufacture of thin film solar cells.[43]
- Used in light-emitting diodes (LEDs) and laser diodes based on compound semiconductors such as InGaN, InGaP that are fabricated by Metalorganic Vapor Phase Epitaxy (MOVPE) technology.[44]
- Ultrapure metalorganics of indium include high purity trimethylindium (TMI), which is used as a precursor in III-V compound semiconductors, while it is also used as the semiconductor dopant in II-VI compound semiconductors.[45]
- One of many substitutes for mercury in alkaline batteries to prevent the zinc from corroding and releasing hydrogen gas.[46]
Metal and alloys

- Very small amounts used in aluminium alloy sacrificial anodes (for salt water applications) to prevent passivation of the aluminium.
- To bond gold electrical test leads to superconductors, indium is used as a conducting adhesive and applied under a microscope with precision tweezers.[47]
- In the form of a wire it is used as a vacuum seal and a thermal conductor in cryogenics and ultra-high vacuum applications. For example, in manufacturing gaskets which deform to fill gaps.[48]
- Used as a calibration material for Differential scanning calorimetry.[49]
- It is an ingredient in the gallium-indium-tin alloy Galinstan, which is liquid at room temperature while not being toxic like mercury.[50]
Other uses
- Indium tin oxide is used as a light filter in low-pressure sodium vapor lamps. The infrared radiation is reflected back into the lamp, which increases the temperature within the tube and therefore improves the performance of the lamp.[39]
- Indium's melting point of 429.7485 K (156.5985 °C) is a defining fixed point on the international temperature scale ITS-90.[51]
- Indium's high neutron capture cross section for thermal neutrons makes it suitable for use in control rods for nuclear reactors, typically in an alloy containing 80% silver, 15% indium, and 5% cadmium.[52]
- In nuclear engineering, the (n,n') reactions of 113In and 115In are used to determine magnitudes of neutron fluxes.[53]
- Indium is also used as a thermal interface material by personal computer enthusiasts in the form of pre-shaped foil sheets fitted between the heat-transfer surface of a microprocessor and its heat sink. The application of heat partially melts the foil and allows the indium metal to fill in any microscopic gaps and pits between the two surfaces, removing any insulating air pockets that would otherwise compromise heat transfer efficiency.[54]
- 111In emits gamma radiation and is used in indium leukocyte imaging, or indium scintigraphy, a technique of medical imaging that is particularly helpful in differentiating conditions such as osteomyelitis from decubitus ulcers for assessment of route and duration of antibiotic therapy.[55] Indium leukocyte scintigraphy has many applications, including early phase drug development, and the monitoring of activity of white blood cells. For the test, blood is taken from the patient, white cells removed, labeled with the radioactive 111In, then re-injected back into the patient. Gamma imaging will then reveal any areas of on-going white cell localization such as new and developing areas of infection.
Precautions and health issues
Pure indium in metal form is considered nontoxic by most sources. In the welding and semiconductor industries, where indium exposure is relatively high, there have been no reports of any toxic side-effects. Indium compounds, like aluminum compounds, complex with hydroxyls to form insoluble salts in basic conditions, and are thus not well-absorbed from food, giving them fairly low oral toxicty. Soluble indium(III) is toxic when delivered parenterally, however, causing damage primarily to the kidney (both inner and outer parts), but additionally to heart and liver, and may be teratogenic.[56] Other indium compounds are toxic when administered outside the gastrointestinal tract: for example, anhydrous indium trichloride (InCl3) and indium phosphide (InP) are quite toxic when delivered into the lungs (the latter is a suspected carcinogen).[57][58] Occupational exposure to indium compounds was associated with PAP, cholesterol ester crystals and granulomas, pulmonary fibrosis, emphysema, and pneumothoraces. The available evidence suggests exposure to indium compounds causes a novel lung disease that may begin with PAP and progress to include fibrosis and emphysema, and, in some cases, premature death [59]
See also
References
- ↑ Mangum, B W (1989). "Determination of the Indium Freezing-point and Triple-point Temperatures". Metrologia 26 (4): 211. Bibcode:1989Metro..26..211M. doi:10.1088/0026-1394/26/4/001.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ 3.0 3.1 3.2 3.3 Alfantazi, A. M.; Moskalyk, R. R. (2003). "Processing of indium: a review". Minerals Engineering 16 (8): 687–694. doi:10.1016/S0892-6875(03)00168-7.
- ↑ Binder, Harry H. (1999). Lexicon der chemischen Elemente (in German). S. Hirzel Verlag. ISBN 3-7776-0736-3.
- ↑ 5.0 5.1 Dean, John A. (523). Lange's handbook of chemistry (Fifteenth edition). McGraw-Hill, Inc. ISBN 0-07-016190-9.
- ↑ (German) Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils (1985). "Thallium". Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 892–893. ISBN 3-11-007511-3.
- ↑ 7.0 7.1 (Russian) Bleshinsky, S. V.; Abramova, V. F. (1958). Химия индия. Frunze. p. 252.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 8.20. ISBN 1439855110.
- ↑ (Russian) Bleshinsky, S. V.; Abramova, V. F. (1958). Химия индия. Frunze. p. 301.
- ↑ Fischer, E. O.; Hofmann, H. P. (1957). "Metall-cyclopentadienyle des Indiums". Angewandte Chemie (in German) 69 (20): 639–640. doi:10.1002/ange.19570692008.
- ↑ 11.0 11.1 Sinclair, Ian; Worrall, Ian J. (1982). "Neutral complexes of the indium dihalides". Canadian Journal of Chemistry 60 (6): 695–698. doi:10.1139/v82-102.
- ↑ Beck, Horst Philipp; Wilhelm, Doris (1991). "In7Cl9—A New"Old" Compound in the System In-Cl". Angewandte Chemie International Edition in English 30 (7): 824–825. doi:10.1002/anie.199108241.
- ↑ Dronskowski, Richard (1995). "Synthesis, Structure, and Decay of In4Br7". Angewandte Chemie International Edition in English 34 (10): 1126–1128. doi:10.1002/anie.199511261.
- ↑ Audi, Georges; Bersillon, O.; Blachot, J.; Wapstra, A.H. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A (Atomic Mass Data Center) 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ Boothroyd, A. I. (2006). "Heavy elements in stars". Science 314 (5806): 1690–1691. doi:10.1126/science.1136842. PMID 17170281.
- ↑ "Elements, Terrestrial Abundance". www.daviddarling.info. Retrieved 2007-04-14.
- ↑ Sutherland, J. K. (1971). "A second occurrence of dzhalindite". The Canadian Mineralogist 10 (5): 781–786.
- ↑ "Mineral Commodities Summary 2007: Indium" (PDF). United States Geological Survey. Retrieved 2007-12-26.
- ↑ 19.0 19.1 "Indium and Gallium Supply Sustainability September 2007 Update" (PDF). 22nd EU PV Conference, Milan, Italy. Retrieved 2007-12-26.
- ↑ "Indium Price Supported by LCD Demand and New Uses for the Metal". September 6, 2009.
- ↑ "Top World Silver Producers" (PDF). World Silver Survey 2007.
- ↑ Reich, F.; Richter, T. (1863). "Ueber das Indium". Journal für Praktische Chemie (in German) 90 (1): 172–176. doi:10.1002/prac.18630900122.
- ↑ Venetskii, S. (1971). "Indium". Metallurgist 15 (2): 148–150. doi:10.1007/BF01088126.
- ↑ Reich, F.; Richter, T. (1864). "Ueber das Indium". Journal für Praktische Chemie (in German) 92 (1): 480–485. doi:10.1002/prac.18640920180.
- ↑ 25.0 25.1 Schwarz-Schampera, Ulrich; Herzig, Peter M. (2002). Indium: Geology, Mineralogy, and Economics. Springer. ISBN 978-3-540-43135-0.
- ↑ French, Sidney J. (1934). "A story of indium". Journal of Chemical Education 11 (5): 270. Bibcode:1934JChEd..11..270F. doi:10.1021/ed011p270.
- ↑ Schwarz-Schampera, Ulrich; Herzig, Peter M; Rohstoffe, Bundesanstalt für Geowissenschaften und (2002-06-10). "history". Indium: Geology, mineralogy, and economics. p. 1. ISBN 978-3-540-43135-0.
- ↑ Weeks, R.A. (1973). "Gallium, germanium, and indium". In Brobst, Donald Albert; Pratt, Walden P. Mines and mineral resources. pp. 237–246.
- ↑ Jorgenson, John D.; George, Micheal W. "Mineral Commodity Profile Indium". United States Geological Survey.
- ↑ "Canadian Minerals Yearbook". 2007. Archived from the original on 2011-09-27.
- ↑ "Recycling Rates of Metals: A Status Report". United Nations Environmental Programme. Retrieved August 2, 2011.
- ↑ Tolcin, Amy C. (2013) Indium. USGS Mineral Commodity Summaries.
- ↑ "Malku Khota Updated Preliminary Economic Assessment, May 2011". South American Silver Corp. Archived from the original on 2012-01-19.
- ↑ Wright, Philip (1996). "Mineral and Metal Commodity Review: Tin". Natural Resources Canada. Retrieved 2011-06-01.
- ↑ "Indium Price Supported by LCD Demand and New Uses for the Metal" (PDF). Geology.com. Retrieved 2007-12-26.
- ↑ "USGS Mineral Commodity Summaries 2011". USGS and USDI. Retrieved August 2, 2011.
- ↑ "How much is left?". ScientificAmerican. Retrieved 16 January 2013.
- ↑ Tolcin, Amy C. "Mineral Yearbook 2007: Indium" (PDF). United States Geological Survey. Retrieved 200-02-03.
- ↑ 39.0 39.1 Downs, Anthony John (1993). Chemistry of Aluminium, Gallium, Indium, and Thallium. Springer. pp. 89 and 106. ISBN 978-0-7514-0103-5.
- ↑ "The Electroluminescent Light Sabre". Nanotechnology News Archive. Azonano. June 2, 2005. Retrieved 2007-08-29.
- ↑ Bachmann, K. J. (1981). "Properties, Preparation, and Device Applications of Indium Phosphide". Annual Review of Materials Science 11: 441–484. Bibcode:1981AnRMS..11..441B. doi:10.1146/annurev.ms.11.080181.002301.
- ↑ Bhuiyan, Ghani; Hashimoto, Akihiro; Yamamoto, Akioare (2003). "Indium nitride (InN): A review on growth, characterization, and properties". Journal of Applied Physics 94 (5): 2779. Bibcode:2003JAP....94.2779B. doi:10.1063/1.1595135.
- ↑ Powalla, M.; Dimmler, B. (2000). "Scaling up issues of CIGS solar cells". Thin Solid Films. 361–362: 540–546. Bibcode:2000TSF...361..540P. doi:10.1016/S0040-6090(99)00849-4.
- ↑ Schubert, E. Fred (2003). Light-Emitting Diodes. Cambridge University Press. p. 16. ISBN 0-521-53351-1.
- ↑ Shenai, Deodatta V.; Timmons, Michael L.; DiCarlo Jr., Ronald L.; Marsman, Charles J. (2004). "Correlation of film properties and reduced impurity concentrations in sources for III/V-MOVPE using high-purity trimethylindium and tertiarybutylphosphine". Journal of Crystal Growth 272 (1–4): 603–608. Bibcode:2004JCrGr.272..603S. doi:10.1016/j.jcrysgro.2004.09.006.
- ↑ Geological Survey (U.S.) (2010). Minerals Yearbook, 2008, V. 1, Metals and Minerals. Government Printing Office. pp. 35–2. ISBN 978-1-4113-3015-3.
- ↑ Rabilloud, Guy (1997). High-performance Polymers: Conductive adhesives. Editions TECHNIP. p. 263. ISBN 2-7108-0716-5.
- ↑ Weissler, G. L., ed. (1990). Vacuum physics and technology. San Diego: Acad. Press. p. 296. ISBN 978-0-12-475914-5.
- ↑ Reading, Mike; Hourston, Douglas J. (2006). Modulated temperature differential scanning calorimetry. Springer. p. 245. ISBN 978-1-4020-3749-8.
- ↑ Surmann, P; Zeyat, H (Nov 2005). "Voltammetric analysis using a self-renewable non-mercury electrode". Analytical and Bioanalytical Chemistry 383 (6): 1009–13. doi:10.1007/s00216-005-0069-7. PMID 16228199.
- ↑ Preston-Thomas, H. (1990). "Procès-Verbaux du Comité International des Poids et Mesures". Metrologia 27 (1): 3–10. Bibcode:1990Metro..27....3P. doi:10.1088/0026-1394/27/1/002.
- ↑ Scoullos, Michael J (2001-12-31). "Other types of cadmium alloys". Mercury, cadmium, lead: handbook for sustainable heavy metals policy and regulation. p. 222. ISBN 978-1-4020-0224-3.
- ↑ Berger, Harold; National Bureau Of Standards, United States; Committee E-7 On Nondestructive Testing, American Society for Testing and Materials (1976). "Image Detectors for Other Neutron Energies". Practical applications of neutron radiography and gaging: a symposium. pp. 50–51.
- ↑ Tong, Xingcun Colin (2011). Advanced Materials for Thermal Management of Electronic Packaging. Springer. p. 323. ISBN 978-1-4419-7759-5.
- ↑ Van Nostrand, D.; Abreu, S. H.; Callaghan, J. J.; Atkins, F. B.; Stoops, H. C.; Savory, C. G. (May 1988). "In-111-labeled white blood cell uptake in noninfected closed fracture in humans: prospective study". Radiology (Radiological Society of North America, Inc.) 167 (2): 495–498. PMID 3357961. Retrieved July 20, 2011.
- ↑ "Indium". Lenntech.com. 1998.
- ↑ Tanaka, A.; Hirata, M.; Omura, M., (2002). "Pulmonary toxicity of indium-tin oxide and indium phosphide after intratracheal instillations into the lung of hamsters". Journal of the Occupational Health 44 (2): 99–102. doi:10.1539/joh.44.99.
- ↑ Blazka, M. E.; Dixon, D., Haskins, E., Rosenthal, G. J. (1994). "Pulmonary toxicity to intratracheally administered indium trichloride in Fischer 344 rats". Fundamental Applied Toxicology 22 (2): 231–239. doi:10.1006/faat.1994.1027.
- ↑ Cummings, KJ; Nakano, M; Omae, K; Takeuchi, K; Chonan, T; Xiao, YL; Harley, RA; Roggli, VL; Hebisawa, A; Tallaksen, RJ; Trapnell, BC; Day, GA; Saito, R; Stanton, ML; Suarthana, E; Kreiss, K (2012). "Indium lung disease". Chest 141 (6): 1512–21. doi:10.1378/chest.11-1880. PMC 3367484. PMID 22207675.
External links
- Indium at The Periodic Table of Videos (University of Nottingham)
- Reducing Agents > Indium low valent
- NIOSH Pocket Guide to Chemical Hazards (Centers for Disease Control and Prevention)
| Periodic table | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
|
| |||||||||||||||||||||||||||||||||
| Large version | |||||||||||||||||||||||||||||||||
| |||||