Imidazoline
| Imidazoline | |
|---|---|
 | |
| IUPAC name 4,5-Dihydro-1H-imidazole | |
| Identifiers | |
| CAS number | 504-75-6 |
| PubChem | 68156 |
| ChemSpider | 61464 |
| ChEBI | CHEBI:53094 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C3H6N2 |
| Molar mass | 70.09 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Imidazoline (dihydroimidazoles) is a nitrogen-containing heterocycle with formula C3H6N2, derived from imidazole. The ring contains an imine bond, and the carbons at the 4 and 5 positions are singly bonded, rather than doubly bonded for the case of imidazole. Imidazolines are structurally related to guanidines and amidines. According to the position of the double bond, imidazolines can be classified as 2-imidazoline, 3-imidazoline, and 4-imidazoline. Among these, 2-imidazolines are the most important. 2-Imidazolines can be found in some natural products and some commercial pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.[1]

Biological role
Many imidazolines are biologically active.[2] Most bio-active derivatives bear a substituent (aryl or alkyl group) on the carbon between the nitrogen centers. Some generic names include oxymetazoline, xylometazoline, tetrahydrozoline, and naphazoline.
Synthesis of Imidazoline
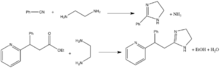
A variety of routes exist for the synthesis of imidazolines. The most common method involves condensation of 1,2-diamines with nitriles and with esters. Alkyl and aryl nitriles react with salts of 1,2-ethanediamine at high temperatures to form 2-substituted 2-imidazolines and esters can also react with 1,2-ethanediamine to form 2-imidazolines.[3][4]
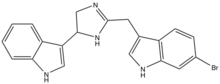
Imidazoline in Natural Products
Imidazoline has been found in various natural products. Natural molecules topsentin D and spongotine B were discovered in several marine sponges. These metabolites have received considerable attention because of their potent properties such as antitumor, antiviral, and anti-inflammatory activities.[5]
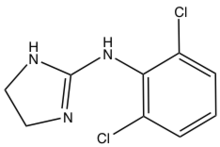
Pharmaceutical Applications
2-imidazolines have been investigated as antihyperglycemic, anti-inflammatory, antihypertensive, antihypercholesterolemic, and antidepressant reagents.[6][7]The imidazoline-containing drug clonidine is used alone or in combination with other medications to treat high blood pressure. It is also used in the treatment of dysmenorrhea, hypertensive crisis, Tourette's syndrome and attention deficit hyperactivity disorder (ADHD).[8]
Homogenous Catalysis
As a structural analogue of 2-oxazolines, 2-imidazolines have been developed as ligands in coordination chemistry. The substitutions on the nitrogen atom in the imidazoline ring provide opportunities for fine-tuning the electronic and steric properties. Some of the complexes function as catalysts for Suzuki–Miyaura couplings, Mizoroki–Heck reactions, Diels–Alder reactions, asymmetric allylic substitution, [3,3] sigmatropic rearrangement, Henry reactions, etc.[9]


Industrial Applications
Imidazoline derivatives have extensive applications in surfactant in small proportions for improving detergent qualities for various purposes (fabric softeners, hair and fabric conditioning).[10]
Imidazolines as Precursors of Imidazoles
Imidazoles can be prepared from dehydrogenation of imidazolines.[11]
See also
References
- ↑ Liu, H. and Du, D.-M. (2009), Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis. Adv. Synth. Catal., 351: 489–519. doi: 10.1002/adsc.200800797
- ↑ N. MacInnes and S. Duty (2004). "Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway". Br J Pharmacol 143 (8): 952–959. doi:10.1038/sj.bjp.0706019. PMC 1575965. PMID 15545290.
- ↑ Liu, H. and Du, D.-M. (2009), Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis. Adv. Synth. Catal., 351: 489–519. doi: 10.1002/adsc.200800797
- ↑ Crouch, R. D. (2009), Synthetic routes toward 2-substituted 2-imidazolines. Tetrahedron, 65, 2387–2397. doi: 10.1016/j.tet.2008.12.022
- ↑ Guinchard, X., Valle´e, Y., Denis, J. N. (2007), Total Synthesis of Marine Sponge Bis(indole) Alkaloids of the Topsentin Class. J. Org. Chem, 72(10), 3972-3975. doi: 10.1021/jo070286r
- ↑ Liu, H. and Du, D.-M. (2009), Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis. Adv. Synth. Catal., 351: 489–519. doi: 10.1002/adsc.200800797
- ↑ Dardonville, C. and Rozas, I. (2004), Imidazoline binding sites and their ligands: An overview of the different chemical structures. Med. Res. Rev., 24: 639–661. doi: 10.1002/med.20007
- ↑ “Clonidine”, Pubmed Health, http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000623/
- ↑ Liu, H. and Du, D.-M. (2009), Recent Advances in the Synthesis of 2-Imidazolines and Their Applications in Homogeneous Catalysis. Adv. Synth. Catal., 351: 489–519. doi: 10.1002/adsc.200800797
- ↑ Tyagi, R., Tyagi, V. K., Pandey, S. K. (2007), Imidazoline and its derivatives: an overview. J. Oleo. Sci., 56(5), 211-222. doi: 10.5650/jos.56.21
- ↑ Ishihara, M., Togo, H. (2006), An Efficient Preparation of 2-Imidazolines and Imidazoles from Aldehydes with Molecular Iodine and (Diacetoxyiodo)benzene. Synlett, 227-230. doi: 10.1055/s-2005-923604