Hysterectomy
| Hysterectomy | |
|---|---|
| Intervention | |
| ICD-9-CM | 68.9 |
| MeSH | D007044 |
| MedlinePlus | 002915 |
Hysterectomy is the surgical removal of the uterus. It may also involve removal of the cervix, ovaries, fallopian tubes and other surrounding structures.
Usually performed by a gynecologist, hysterectomy may be total (removing the body, fundus, and cervix of the uterus; often called "complete") or partial (removal of the uterine body while leaving the cervix intact; also called "supracervical"). It is the most commonly performed gynecological surgical procedure. In 2003, over 600,000 hysterectomies were performed in the United States alone, of which over 90% were performed for benign conditions.[1] Such rates being highest in the industrialized world has led to the major controversy that hysterectomies are being largely performed for unwarranted and unnecessary reasons.[2]
Removal of the uterus renders the patient unable to bear children (as does removal of ovaries and fallopian tubes) and has surgical risks as well as long-term effects, so the surgery is normally recommended when other treatment options are not available or have failed. It is expected that the frequency of hysterectomies for non-malignant indications will fall as there are good alternatives in many cases.[3]
Oophorectomy (removal of ovaries) is frequently done together with hysterectomy to decrease the risk of ovarian cancer. However, recent studies have shown that prophylactic oophorectomy without an urgent medical indication decreases a woman's long-term survival rates substantially and has other serious adverse effects.[4] This effect is not limited to pre-menopausal women; even women who have already entered menopause were shown to have experienced a decrease in long-term survivability post-oophorectomy.[5]
Incidence
Canada
In Canada, the number of hysterectomies between 2008 and 2009 was almost 47,000. The national rate in for the same timeline was 338 per 100,000 population, down from 484 per 100,000 in 1997. The reasons for hysterectomies differed depending on whether the woman was living in an urban or rural location. Urban women opted for hysterectomies due to uterine fibroids and rural women had hysterectomies mostly for menstrual disorders.[6]
United States
According to the National Center for Health Statistics, of the 617,000 hysterectomies performed in 2004, 73% also involved the surgical removal of the ovaries. In the United States, 1 in 3 women can be expected to have a hysterectomy by age 60.[7] There are currently an estimated 22 million people in the United States who have undergone this procedure.
United Kingdom
In the UK, 1 in 5 women are likely to have a hysterectomy by the age of 60, and ovaries are removed in about 20% of hysterectomies.[8]
Indications
Hysterectomy is a major surgical procedure that has risks and benefits, and affects a woman's hormonal balance and overall health for the rest of her life. Because of this, hysterectomy is normally recommended as a last resort to remedy certain intractable uterine/reproductive system conditions. Such conditions include, but are not limited to:
- Certain types of reproductive system cancers (uterine, cervical, ovarian, endometrium) or tumors, including uterine fibroids that do not respond to more conservative treatment options.[9]
- Severe and intractable endometriosis (growth of the uterine lining outside the uterine cavity) and/or adenomyosis (a form of endometriosis, where the uterine lining has grown into and sometimes through the uterine wall musculature), after pharmaceutical or other surgical options have been exhausted.[9]
- Chronic pelvic pain, after pharmaceutical or other surgical options have been exhausted.[9]
- Postpartum to remove either a severe case of placenta praevia (a placenta that has either formed over or inside the birth canal) or placenta percreta (a placenta that has grown into and through the wall of the uterus to attach itself to other organs), as well as a last resort in case of excessive obstetrical haemorrhage.[10]
- Several forms of vaginal prolapse.[9]
Occasionally, women express a desire to undergo an elective hysterectomy—that is, a hysterectomy for reasons other than the resolution of reproductive system conditions or illnesses. Some of the conditions under which a person may request to have a hysterectomy (or have one requested for her if the woman is incapable of making the request) for non-illness reasons include:
- Prophylaxis against certain reproductive system cancers, especially if there is a strong family history of reproductive system cancers (especially breast cancer in conjunction with BRCA1 or BRCA2 mutation), or as part of recovery from such cancers.
- Part of overall gender transition for trans men.[11]
- Severe developmental disabilities, though this treatment is controversial at best, and specific cases of sterilization due to developmental disabilities have been found by state-level Supreme Courts to violate the patient's constitutional and common law rights.[12]
Types

Hysterectomy, in the literal sense of the word, means merely removal of the uterus. However other organs such as ovaries, fallopian tubes and the cervix are very frequently removed as part of the surgery.
- Radical hysterectomy : complete removal of the uterus, cervix, upper vagina, and parametrium. Indicated for cancer. Lymph nodes, ovaries and fallopian tubes are also usually removed in this situation, such as in Wertheim's hysterectomy.[13]
- Total hysterectomy : Complete removal of the uterus and cervix, with or without oophorectomy.
- Subtotal hysterectomy : removal of the uterus, leaving the cervix in situ.
Subtotal (supracervical) hysterectomy was originally proposed with the expectation that it may improve sexual functioning after hysterectomy, it has been postulated that removing the cervix causes excessive neurologic and anatomic disruption, thus leading to vaginal shortening, vaginal vault prolapse, and vaginal cuff granulations. These theoretical advantages were not confirmed in practice, but other advantages over total hysterectomy emerged. The principal disadvantage is that risk of cervical cancer is not eliminated and women may continue cyclical bleeding (although substantially less than before the surgery). These issues were addressed in a systematic review of total versus supracervical hysterectomy for benign gynecological conditions, which reported the following findings:[14]
- There was no difference in the rates of incontinence, constipation, measures of sexual function or alleviation of pre-surgery symptoms.
- Length of surgery and amount of blood lost during surgery were significantly reduced during supracervical hysterectomy compared to total hysterectomy, but there was no difference in post-operative transfusion rates.
- Febrile morbidity was less likely and ongoing cyclic vaginal bleeding one year after surgery was more likely after supracervical hysterectomy.
- There was no difference in the rates of other complications, recovery from surgery, or readmission rates.
In the short-term, randomized trials have shown that cervical preservation or removal does not affect the rate of subsequent pelvic organ prolapse.[15]
Supracervical hysterectomy does not eliminate the possibility of having cervical cancer since the cervix itself is left intact and may be contraindicated in women with increased risk of this cancer, regular pap smears to check for cervical dysplasia or cancer are still needed.[16][17]
Technique
Hysterectomy can be performed in different ways. The oldest known technique is abdominal incision. Subsequently the vaginal (performing the hysterectomy through the vaginal canal) and later laparoscopic vaginal (with additional instruments inserted through a small hole, frequently close to the navel) techniques were developed.
Most hysterectomies in the United States are done via laparotomy (abdominal incision, not to be confused with laparoscopy). A transverse (Pfannenstiel) incision is made through the abdominal wall, usually above the pubic bone, as close to the upper hair line of the individual's lower pelvis as possible, similar to the incision made for a caesarean section. This technique allows doctors the greatest access to the reproductive structures and is normally done for removal of the entire reproductive complex. The recovery time for an open hysterectomy is 4–6 weeks and sometimes longer due to the need to cut through the abdominal wall. Historically, the biggest problem with this technique were infections, but infection rates are well-controlled and not a major concern in modern medical practice. An open hysterectomy provides the most effective way to explore the abdominal cavity and perform complicated surgeries. Before the refinement of the vaginal and laparoscopic vaginal techniques it was also the only possibility to achieve subtotal hysterectomy, meanwhile vaginal route is the preferable technique in most circumstances.[18][19]
Vaginal hysterectomy is performed entirely through the vaginal canal and has clear advantages over abdominal surgery such as fewer complications, shorter hospital stays and shorter healing time. Abdominal hysterectomy, the most common method, is used in cases such as after caesarean delivery, when the indication is cancer, when complications are expected or surgical exploration is required.
With the development of the laparoscopic techniques in the 1970-1980s, the "laparoscopic-assisted vaginal hysterectomy" (LAVH) has gained great popularity among gynecologists because compared with the abdominal procedure it is less invasive and the post-operative recovery is much faster. It also allows better exploration and slightly more complicated surgeries than the vaginal procedure. LAVH begins with laparoscopy and is completed such that the final removal of the uterus (with or without removing the ovaries) is via the vaginal canal. Thus, LAVH is also a total hysterectomy, the cervix must be removed with the uterus.
The "laparoscopic-assisted supracervical hysterectomy" (LASH) was later developed to remove the uterus without removing the cervix using a morcellator which cuts the uterus into small pieces that can be removed from the abdominal cavity via the laparoscopic ports.
Total laparoscopic hysterectomy (TLH) was developed in the early 90s by Prabhat K. Ahluwalia in Upstate New York.[20] TLH is performed solely through the laparoscopes in the abdomen, starting at the top of the uterus, typically with a uterine manipulator. The entire uterus is disconnected from its attachments using long thin instruments through the "ports". Then all tissue to be removed is passed through the small abdominal incisions.
Supracervical (subtotal) laparoscopic hysterectomy (LSH) is performed similar to the total laparoscopic surgery but the uterus is amputated between the cervix and fundus.
Dual-port laparoscopy is a form of laparoscopic surgery using two 5 mm midline incisions: the uterus is detached through the two ports and removed through the vagina.[21][22]
"Robotic hysterectomy" is a variant of laparoscopic surgery using special remotely controlled instruments that allow the surgeon finer control as well as three-dimensional magnified vision.[23]
-

uterus before hysterectomy
-

laparoscopical hysterectomy
-
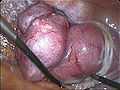
transvaginal extraction of the uterus in total laparoscopical hysterectomy
-

cervical stump (white) after removal of the uterine corpus at laparoscopic supracervical hysterectomy
-
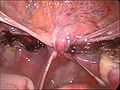
end of an laparoscopical hysterectomy
Comparison of techniques
The abdominal technique is very often applied in difficult circumstances or when complications are expected. Given these circumstances the complication rate and time required for surgery compares very favorably with other techniques, however time required for healing is much longer.
Vaginal hysterectomy was shown to be superior to LAVH and some types of laparoscopic surgery (sufficient data was not available for all types of laparoscopic surgery), causing fewer short- and long-term complications, more favorable effect on sexual experience with shorter recovery times and fewer costs.[24][25][26]
A Cochrane review from 2009 recommends vaginal hysterectomy over other variants where possible. Laparoscopic surgery offers certain advantages when vaginal surgery is not possible but has also the disadvantage of significantly longer time required for the surgery.[27]
In direct comparison of abdominal (laparotomic) and laparoscopic techniques laparoscopic surgery causes longer operation time and substantially higher rate of major complications while offering much quicker healing.[27][28]
Vaginal hysterectomy is the only available option that is feasible without total anaesthesia or in outpatient settings (although so far doing it without anesthesia is recommended only in exceptional cases).
Time required for completion of surgery in the eVAL trial is reported as following:[28]
- abdominal 55.2 minutes average, range 19-155
- vaginal 46.6 minutes average, range 14-168
- laparoscopic (all variants) 82.5 minutes average, range 10-325 (combined data from both trial arms)
The great majority of hysterectomies for benign indications can be performed by the vaginal technique with clear advantages.[18][19]
Morcellation is widely used especially in laparoscopic techniques and sometimes for the vaginal technique but appears to be associated with a considerable risk of spreading benign or malignant tumors.[29][30]
Robotic surgery has very similar clinical outcomes like standard laparoscopic or vaginal techniques. Previously observed marginal advantages could not be confirmed, only differences in hospital stay and cost remains statistically significant.[31] [32][33][34] Robotic surgery does not benefit women with benign gynaecological disease[35] and concerns over widespread misleading marketing claims have been raised.[36]
Comparison of long term results and complication rates
Hysterectomy by abdominal laparotomy is correlated with much higher incidence of intestinal adhesions than other techniques.[37]
Benefits
Hysterectomy is usually performed for serious conditions and is highly effective in curing those conditions.
The Maine Women Health Study of 1994 followed for 12 months time approximately 800 women with similar gynecological problems (pelvic pain, urinary incontinence due to uterine prolapse, severe endometriosis, excessive menstrual bleeding, large fibroids, painful intercourse), around half of whom had a hysterectomy and half of whom did not. The study found that a substantial number of those who had a hysterectomy had marked improvement in their symptoms following hysterectomy, as well as significant improvement in their overall physical and mental health one year out from their surgery. The study concluded that for those who have intractable gynecological problems that had not responded to non-surgical intervention, hysterectomy may be beneficial to their overall health and wellness. Somewhat surprisingly, ovarian cancer risk after hysterectomy appears to be substantially lowered even when the ovaries are preserved.[38]
Adverse effects
Hysterectomy has like any other surgery certain risks and side effects.
Mortality and surgical risks
Short term mortality (within 40 days of surgery) is usually reported in the range of 1–6 cases per 1000 when performed for benign causes. Risks for surgical complications are presence of fibroids, younger age (vascular pelvis with higher bleeding risk and larger uterus), dysfunctional uterine bleeding and parity.[39]
The mortality rate is several times higher when performed in patients that are pregnant, have cancer or other complications.[40]
Long term effect on all case mortality is relatively small. Women under the age of 45 years have a significantly increased long term mortality that is believed to be caused by the hormonal side effects of hysterectomy and prophylactic oophorectomy.[41]
Approximately 35% of women after hysterectomy undergo another related surgery within 2 years.
Ureteral injury is not uncommon and can range from 2.2% to 3% depending on whether the modality is abdominal, laparoscopic, or vaginal. The injury usually occurs in the distal ureter close to the infundibulopelvic ligament or as a ureter crosses below the uterine artery, often from blind clamping and ligature placement to control hemorrhage.[42]
Convalescence
Hospital stay is 3 to 5 days or more for the abdominal procedure and between 2 to 3 days for vaginal or laparoscopically assisted vaginal procedures.
Time for full recovery is very long and largely independent on the procedure that was used. Depending on the definition of "full recovery" 3 to 12 months have been reported. Serious limitations in everyday activities are expected for a minimum of 4 months.
Unintended oophorectomy and premature ovarian failure
Removal of one or both ovaries is performed in a substantial number of hysterectomies that were intended to be ovary sparing.[43] The general extraction by surgery of an ovary and a fallopian tube is called unilateral salpingo-oophorectomy, but if both pairs of ovaries and fallopian tubes are surgically removed the process is called a bilateral salpingo-oophorectomy. The procedure is carried out to treat ovarian cancers or other gynecological cancers, also pelvic inflammatory disease or relative infections. In some instances the extraction of one or both ovaries is recommended to treat a condition called endometriosis, when the uterus lining (endometrium) grows on the outside of the uterus; typically around the pelvic organs.
This operation may also be carried out if a woman is diagnosed with a fallopian tube pregnancy (an ectopic pregnancy), and the pregnancy cannot be removed by a salpingostomy (removing the pregnancy by entering the fallopian tube with an incision).
A woman may be able to conceive in the future if only one of her ovaries and one fallopian tube is removed. The woman will be permanently infertile if both are removed. This surgery is normally combined with a hysterectomy, in other words the complete removal of the uterus.
Several doctors have surmised that the removal of women’s ovaries after the age of 40 as they approached menopause would eliminate the risk of ovarian cancer. When women stopped releasing eggs and producing estrogen risks are reduced and menopause is only accelerated by a few years.
The general attitude towards a routine salpingooophorectomy started to change around the 1990s. The cases of women who had no family history of the problem was less than 1%. Furthermore, there was the increased risk of cardiovascular disease and a speeding up of osteoporosis unless women underwent a course of hormone replacement therapy.
The average onset age of menopause in those who underwent hysterectomy is 3.7 years earlier than average even when the ovaries are preserved.[44] This has been suggested to be due to the disruption of blood supply to the ovaries after a hysterectomy or due to missing endocrine feedback of the uterus. The function of the remaining ovaries is significantly affected in about 40% of women, some of them even require hormone replacement treatment. Surprisingly, a similar and only slightly weaker effect has been observed for endometrial ablation which is often considered as an alternative to hysterectomy.
A substantial number of women develop benign ovarian cysts after a hysterectomy.[45]
Effects on sexual life and pelvic pain
After hysterectomy for benign indications the majority of women report improvement in sexual life and pelvic pain. A smaller share of women report worsening of sexual life and other problems. The picture is significantly different for hysterectomy performed for malignant reasons, the procedure is often more radical with substantial side effects.[46][47] A proportion of patients who undergo a hysterectomy for chronic pelvic pain continue to suffer from pelvic pain after a hysterectomy and develop dyspareunia (painful sexual intercourse).[48]
Premature menopause and its effects
Estrogen levels fall sharply when the ovaries are removed, removing the protective effects of estrogen on the cardiovascular and skeletal systems. This condition is often referred to as "surgical menopause", although it is substantially different from a naturally occurring menopausal state; the former is a sudden hormonal shock to the body that causes rapid onset of menopausal symptoms such as hot flashes, while the latter is a gradually occurring decrease of hormonal levels over a period of years with uterus intact and ovaries able to produce hormones even after the cessation of menstrual periods.
When only the uterus is removed there is a three times greater risk of cardiovascular disease. If the ovaries are removed the risk is seven times greater. Several studies have found that osteoporosis (decrease in bone density) and increased risk of bone fractures are associated with hysterectomies.[49][50][51][52][53][54] This has been attributed to the modulatory effect of estrogen on calcium metabolism and the drop in serum estrogen levels after menopause can cause excessive loss of calcium leading to bone wasting.
Hysterectomies have also been linked with higher rates of heart disease and weakened bones. Those who have undergone a hysterectomy with both ovaries removed typically have reduced testosterone levels as compared to those left intact.[43] Reduced levels of testosterone in women is predictive of height loss, which may occur as a result of reduced bone density,[55] while increased testosterone levels in women are associated with a greater sense of sexual desire.[56]
Oophorectomy before the age of 45 is associated with a fivefold mortality from neurologic and mental disorders.[57]
Urinary incontinence and vaginal prolapse
Urinary incontinence and vaginal prolapse are well known adverse effects that develop with high frequency a very long time after the surgery. Typically, those complications develop 10–20 years after the surgery.[58] For this reason exact numbers are not known, and risk factors are poorly understood. It is also unknown if the choice of surgical technique has any effect. It has been assessed that the risk for urinary incontinence is approximately doubled within 20 years after hysterectomy. One long term study found a 2.4 fold increased risk for surgery to correct urinary stress incontinence following hysterectomy[59][60]
The risk for vaginal prolapse depends on factors such as number of vaginal deliveries, the difficulty of those deliveries, and the type of labor.[61] Overall incidence is approximately doubled after hysterectomy.[62]
Adhesion formation and bowel obstruction
The formation of postoperative adhesions is a particular risk after hysterectomy because of the extent of dissection involved as well the fact the hysterectomy wound is in the most gravity-dependent part of the pelvis into which a loop of bowel may easily fall.[63] In one review, incidence of small bowel obstruction due to intestinal adhesion were found to be 15.6% in non-laparoscopic total abdominal hysterectomies vs. 0.0% in laparopscopic hysterecomies.[37]
Other rare problems
Hysterectomy may cause an increased risk of the relatively rare renal cell carcinoma. The increased risk is particularly pronounced for young women, the risk was lower after vaginally performed hysterectomies.[64] Hormonal effects or injury of the ureter were considered as possible explanations.[65][66] In some cases the renal cell carcinoma may be a manifestation of an undiagnosed hereditary leiomyomatosis and renal cell cancer syndrome.
Removal of the uterus without removing the ovaries can produce a situation that on rare occasions can result in ectopic pregnancy due to an undetected fertilization that had yet to descend into the uterus before surgery. Two cases have been identified and profiled in an issue of the Blackwell Journal of Obstetrics and Gynecology; over 20 other cases have been discussed in additional medical literature.[67]
Alternatives
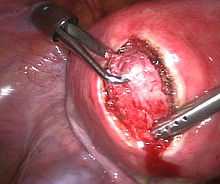

Depending on the indication there are alternatives to hysterectomy :
Heavy bleeding
Levonorgestrel intrauterine devices are highly effective at controlling dysfunctional uterine bleeding (DUB) or menorrhagia and should be considered before any surgery.[68]
Menorrhagia (heavy or abnormal menstrual bleeding) may also be treated with the less invasive endometrial ablation which is an outpatient procedure in which the lining of the uterus is destroyed with heat, mechanically or by radio frequency ablation. Endometrial ablation greatly reduces or entirely eliminates monthly bleeding in ninety percent of patients with DUB. It is not effective for patients with very thick uterine lining or uterine fibroids.[69]
Uterine fibroids
Levonorgestrel intrauterine devices are highly effective in limiting menstrual blood flow and improving other symptoms. Side effects are typically very moderate because the levonorgestrel (a progestin) is released in low concentration locally. There is now substantial evidence that Levongestrel-IUDs provide good symptomatic relief for women with fibroids.[70]
Uterine fibroids may be removed and the uterus reconstructed in a procedure called "myomectomy." A myomectomy may be performed through an open incision, laparoscopically or through the vagina (hysterescopy).[71]
Uterine artery embolization (UAE) is a minimally invasive procedure for treatment of uterine fibroids. Under local anesthesia a catheter is introduced into the femoral artery at the groin and advanced under radiographic control into the uterine arterty. A mass of microspheres or polyvinyl alcohol (PVA) material (an embolus) is injected into the uterine arteries in order to block the flow of blood through those vessels. The restriction in blood supply usually results in significant reduction of fibroids and improvement of heavy bleeding tendency. The 2012 Cochrane review comparing hysterectomy and UAE did not find any major advantage for either procedure. While UAE is associated with shorter hospital stay and a more rapid return to normal daily activities, it was also associated with a higher risk for minor complications later on. There were no differences between UAE and hysterectomy with regards to major complications.[72]
Uterine fibroids can be removed with a non-invasive procedure called Magnetic Resonance guided Focused Ultrasound (MRgFUS).
Prolapse
Prolapse may also be corrected surgically without removal of the uterus.[73]
As part of transitioning from female-to-male
Hysterectomies with bilateral salpingo-oophorectomy are often performed either prior to or as a part of sex reassignment surgery for trans men. Some in the FTM community prefer to have this operation along with hormone replacement therapy in the early stages of their gender transition to avoid complications from heavy testosterone use while still having female-hormone-producing organs in place (e.g. uterine cancer and hormonally induced coronary artery disease) or to remove as many sources of female sex hormones as possible in order to better "pass" during the real life experience portion of their transition.[11] Just as many, however, prefer to wait until they have full "bottom surgery" (removal of female sexual organs and construction of male-appearing external anatomy)[74] to avoid undergoing multiple separate operations.[75]
References
- ↑ Wu, JM; Wechter, ME; Geller, EJ; Nguyen, TV; Visco, AG (2007). "Hysterectomy rates in the United States, 2003". Obstet Gynecol 110 (5): 1091–5. doi:10.1097/01.AOG.0000285997.38553.4b. PMID 17978124.
- ↑ Masters, Coco (2006-07-01). "Are Hysterectomies Too Common?". TIME Magazine. Retrieved 2007-07-17.
- ↑ Bahamondes, L.; Bahamondes, M. V.; Monteiro, I. (2008). "Levonorgestrel-releasing intrauterine system: uses and controversies". Expert Review of Medical Devices 5 (4): 437–445. doi:10.1586/17434440.5.4.437. PMID 18573044.
- ↑ Shuster, L. T.; Gostout, B. S.; Grossardt, B. R.; Rocca, W. A. (2008). "Prophylactic oophorectomy in premenopausal women and long-term health". Menopause International 14 (3): 111–116. doi:10.1258/mi.2008.008016. PMC 2585770. PMID 18714076.
- ↑ Shoupe, D.; Parker, W. H.; Broder, M. S.; Liu, Z.; Farquhar, C.; Berek, J. S. (2007). "Elective oophorectomy for benign gynecological disorders". Menopause 14 (Suppl. 1): 580–585. doi:10.1097/gme.0b013e31803c56a4.
- ↑ "Hysterectomy rates falling: report". CBC News. 2010-05-27. Retrieved 2010-05-28.
- ↑ "Hysterectomy". National Women’s Health Information Center. 2006-07-01. Retrieved 2007-06-07.
- ↑ Gautam Khastgir, John Studd (1998). Hysterectomy and HRT. Taylor & Francis. p. 3. ISBN 978-1-85317-408-7.
- ↑ 9.0 9.1 9.2 9.3 The National Women's Health Information Center (2009-12-15). "Hysterectomy Frequently Asked Questions". Washington, DC: Office of Women's Health, United States Department of Health and Human Services. Retrieved 2011-03-10.
- ↑ Roopnarinesingh R, Fay L, McKenna P (2003). "A 27-year review of obstetric hysterectomy". Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 23 (3): 252–4. doi:10.1080/0144361031000098352. PMID 12850853.
- ↑ 11.0 11.1 Hudson's FTM Resource Guide, "Why Have A Hysterectomy?", retrieved May 8, 2007.
- ↑ Washington (state) Protection and Advocacy System. "Growth Attenuation and Sterilization Procedures – "The Ashley Treatment"". Washington, DC: National Disabilities Rights Network. Retrieved 2011-03-10.
- ↑ encyclopedia.com > Wertheim's hysterectomy Citing: "Wertheim's hysterectomy." A Dictionary of Nursing. 2008. Encyclopedia.com. (October 13, 2010).
- ↑ Lethaby A, Mukhopadhyay A, Naik R (2012). "Total versus subtotal hysterectomy for benign gynaecological conditions". In Lethaby, Anne. Cochrane Database Syst Rev 4 (4): CD004993. doi:10.1002/14651858.CD004993.pub3. PMID 22513925.
- ↑ Thakar, R; Ayers, S; Clarkson, P; Stanton, S; Manyonda, I (2002). "Outcomes after Total versus Subtotal abdominal hysterectomy". N Engl J Med 347 (17): 1318–25. doi:10.1056/NEJMoa013336. PMID 12397189.
- ↑ American Academy of Family Physicians (April 2012). "Five Things Physicians and Patients Should Question". Choosing Wisely: an initiative of the ABIM Foundation (American Academy of Family Physicians). Retrieved August 14, 2012.
- ↑ Consumer Reports; American Academy of Family Physicians (May 2012). "Pap tests: When you need them—and when you don't". Choosing Wisely: an initiative of the ABIM Foundation (Consumer Reports). Retrieved August 17, 2012.
- ↑ 18.0 18.1 Thomas, B.; Magos, A. (2011). "Subtotal hysterectomy and myomectomy - Vaginally". Best Practice & Research Clinical Obstetrics & Gynaecology 25 (2): 133–152. doi:10.1016/j.bpobgyn.2010.11.003. PMID 21185235.
- ↑ 19.0 19.1 Sheth, S. S.; Paghdiwalla, K. P.; Hajari, A. R. (2011). "Vaginal route: A gynaecological route for much more than hysterectomy". Best Practice & Research Clinical Obstetrics & Gynaecology 25 (2): 115–132. doi:10.1016/j.bpobgyn.2010.12.005. PMID 21349773.
- ↑ "Total Laparoscopic Hysterectomy," PK Ahluwalia, J Am Assoc Gynecol Laparosc. 1996 Aug;3(4, Supplement):S1-2. http://www.ncbi.nlm.nih.gov/pubmed/?term=ahluwalia+tlh
- ↑ Lii, S. J.; Becker, S. F.; Danilyants, N. E.; MacKoul, P. J. (2010). "A Novel Approach to Total Laparoscopic Hysterectomy Using Only Two 5mm Ports: Initial Clinical Experience". Journal of Minimally Invasive Gynecology 17 (6): S87. doi:10.1016/j.jmig.2010.08.381.
- ↑ Moawad, G.; Robinson, J. K. (2012). "Dual Port Hysterectomy: A Novel Technique and Initial Experience". Journal of Minimally Invasive Gynecology 19 (6): S86. doi:10.1016/j.jmig.2012.08.620.
- ↑ Medline Plus: Robotic surgery
- ↑ Stovall, T. G.; Summitt Jr, R. (1996). "Laparoscopic Hysterectomy -- is There a Benefit?". New England Journal of Medicine 335 (7): 512–513. doi:10.1056/NEJM199608153350712. PMID 8672159.
- ↑ "Laparoscopic Hysterectomy and Health Care in America – Finding the Balance Between Costs and Outcomes". Retrieved 2010-01-24.
- ↑ Debodinance, P (2001). "Hysterectomy for benign lesions in the north of France: epidemiology and postoperative events". Journal de gynecologie, obstetrique et biologie de la reproduction 30 (2): 151–9. PMID 11319467.
- ↑ 27.0 27.1 Nieboer, T. E.; Johnson, N.; Lethaby, A.; Tavender, E.; Curr, E.; Garry, R.; van Voorst, S.; Mol, B. W. J.; Kluivers, K. B. (2009). "Surgical approach to hysterectomy for benign gynaecological disease". In Kluivers, Kirsten B. Cochrane Database of Systematic Reviews (3): CD003677. doi:10.1002/14651858.CD003677.pub4. PMID 19588344.
- ↑ 28.0 28.1 Garry, R.; Fountain, J.; Mason, S.; Napp, J.; Brown, V.; Hawe, J.; Clayton, R.; Abbott, G.; Phillips, M.; Whittaker, R.; Lilford, S.; Bridgman, J. (2004). "The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy". BMJ (Clinical research ed.) 328 (7432): 129. doi:10.1136/bmj.37984.623889.F6. PMC 314503. PMID 14711749.
- ↑ Seidman, M. A.; Oduyebo, T.; Muto, M. G.; Crum, C. P.; Nucci, M. R.; Quade, B. J. (2012). "Peritoneal Dissemination Complicating Morcellation of Uterine Mesenchymal Neoplasms". In Sullivan, David J. PLoS ONE 7 (11): e50058. doi:10.1371/journal.pone.0050058. PMC 3506532. PMID 23189178.
- ↑ Cucinella, G.; Granese, R.; Calagna, G.; Somigliana, E.; Perino, A. (2011). "Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases". Fertility and Sterility 96 (2): e90–e96. doi:10.1016/j.fertnstert.2011.05.095. PMID 21719004.
- ↑ Wright, J. D.; Ananth, C. V.; Lewin, S. N.; Burke, W. M.; Lu, Y. S.; Neugut, A. I.; Herzog, T. J.; Hershman, D. L. (2013). "Robotically Assisted vs Laparoscopic Hysterectomy Among Women with Benign Gynecologic Disease<alt-title>Prevalence of Robotically Assisted Hysterectomy</alt-title>". JAMA 309 (7): 689–698. doi:10.1001/jama.2013.186. PMID 23423414.
- ↑ Weinberg, L.; Rao, S.; Escobar, P. F. (2011). "Robotic Surgery in Gynecology: An Updated Systematic Review". Obstetrics and Gynecology International 2011: 1. doi:10.1155/2011/852061. PMC 3236390. PMID 22190948.
- ↑ Soto, E.; Lo, Y.; Friedman, K.; Soto, C.; Nezhat, F.; Chuang, L.; Gretz, H. (2011). "Total laparoscopic hysterectomy versus da Vinci robotic hysterectomy: Is using the robot beneficial?". Journal of Gynecologic Oncology 22 (4): 253–259. doi:10.3802/jgo.2011.22.4.253. PMC 3254844. PMID 22247802.
- ↑ Sarlos, D.; Kots, L. A. (2011). "Robotic versus laparoscopic hysterectomy". Current Opinion in Obstetrics and Gynecology 23 (4): 283–288. doi:10.1097/GCO.0b013e328348a26e. PMID 21666467.
- ↑ Liu, H.; Lu, D.; Wang, L.; Shi, G.; Song, H.; Clarke, J. (2012). Robotic surgery for benign gynaecological disease. In Shi, Gang. "Cochrane Database of Systematic Reviews". Cochrane database of systematic reviews (Online) 2: CD008978. doi:10.1002/14651858.CD008978.pub2. PMID 22336855.
- ↑ Schiavone, M. B.; Kuo, E. C.; Naumann, R. W.; Burke, W. M.; Lewin, S. N.; Neugut, A. I.; Hershman, D. L.; Herzog, T. J.; Wright, J. D. (2012). "The commercialization of robotic surgery: Unsubstantiated marketing of gynecologic surgery by hospitals". American Journal of Obstetrics and Gynecology 207 (3): 174.1e1–7. doi:10.1016/j.ajog.2012.06.050. PMID 22835493.
- ↑ 37.0 37.1 Barmparas G, Branco BC, Schnüriger B, Lam L, Inaba K, Demetriades D (October 2010). "The incidence and risk factors of post-laparotomy adhesive small bowel obstruction". J. Gastrointest. Surg. 14 (10): 1619–28. doi:10.1007/s11605-010-1189-8. PMID 20352368.
- ↑ Parker WH. "Hysterectomy—A Gynecologist's Second Opinion". Retrieved 2007-06-07.
- ↑ McPherson, K.; Metcalfe, M.; Herbert, A.; Maresh, M.; Casbard, A.; Hargreaves, J.; Bridgman, S.; Clarke, A. (2004). "Severe complications of hysterectomy: the VALUE study". BJOG : an international journal of obstetrics and gynaecology 111 (7): 688–694. doi:10.1111/j.1471-0528.2004.00174.x. PMID 15198759.
- ↑ Wingo, P. A.; Huezo, C. M.; Rubin, G. L.; Ory, H. W.; Peterson, H. B. (1985). "The mortality risk associated with hysterectomy". American journal of obstetrics and gynecology 152 (7 Pt 1): 803–808. PMID 4025434.
- ↑ Shuster, L. T.; Gostout, B. S.; Grossardt, B. R.; Rocca, W. A. (2008). "Prophylactic oophorectomy in premenopausal women and long-term health". Menopause International 14 (3): 111–116. doi:10.1258/mi.2008.008016. PMC 2585770. PMID 18714076.
- ↑ Santucci, Richard A and Hunter, Craig B (May 21, 2012) Ureteral Trauma. emedicine.medscape.com
- ↑ 43.0 43.1 Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D (2000). "Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study". J. Clin. Endocrinol. Metab. 85 (2): 645–51. doi:10.1210/jc.85.2.645. PMID 10690870.
- ↑ Farquhar CM, Sadler L, Harvey SA, Stewart AW (2005). "The association of hysterectomy and menopause: a prospective cohort study". BJOG : an international journal of obstetrics and gynaecology 112 (7): 956–62. doi:10.1111/j.1471-0528.2005.00696.x. PMID 15957999.
- ↑ Petri Nahás, E.; Pontes, A.; Nahas-Neto, J.; Borges, V.; Dias, R.; Traiman, P. (2005). "Effect of total abdominal hysterectomy on ovarian blood supply in women of reproductive age". Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 24 (2): 169–174. PMID 15661947.
- ↑ Maas, C. P.; Weijenborg, P. T.; Ter Kuile, M. M. (2003). "The effect of hysterectomy on sexual functioning". Annual review of sex research 14: 83–113. PMID 15287159.
- ↑ Komisaruk, B. R.; Frangos, E.; Whipple, B. (2011). "Hysterectomy Improves Sexual Response? Addressing a Crucial Omission in the Literature". Journal of Minimally Invasive Gynecology 18 (3): 288–295. doi:10.1016/j.jmig.2011.01.012. PMC 3090744. PMID 21545957.
- ↑ Gunter, J. (2003). "Chronic Pelvic Pain: An Integrated Approach to Diagnosis and Treatment". Obstetrical & Gynecological Survey 58 (9): 615–623. doi:10.1097/01.OGX.0000083225.90017.01. PMID 12972837.
- ↑ Kelsey JL, Prill MM, Keegan TH, Quesenberry CP, Sidney S (2005). "Risk factors for pelvis fracture in older persons". Am. J. Epidemiol. 162 (9): 879–86. doi:10.1093/aje/kwi295. PMID 16221810.
- ↑ van der Voort DJ, Geusens PP, Dinant GJ (2001). "Risk factors for osteoporosis related to their outcome: fractures". Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 12 (8): 630–8. doi:10.1007/s001980170062. PMID 11580076.
- ↑ Watson NR, Studd JW, Garnett T, Savvas M, Milligan P (1995). "Bone loss after hysterectomy with ovarian conservation". Obstetrics and gynecology 86 (1): 72–7. doi:10.1016/0029-7844(95)00100-6. PMID 7784026.
- ↑ Durães Simões R, Chada Baracat E, Szjenfeld VL, de Lima GR, José Gonçalves W, de Carvalho Ramos Bortoletto C (1995). "Effects of simple hysterectomy on bone loss". Revista paulista de medicina 113 (6): 1012–5. PMID 8731286.
- ↑ Hreshchyshyn MM, Hopkins A, Zylstra S, Anbar M (1988). "Effects of natural menopause, hysterectomy, and oophorectomy on lumbar spine and femoral neck bone densities". Obstetrics and gynecology 72 (4): 631–8. PMID 3419740.
- ↑ Menon RK, Okonofua FE, Agnew JE, et al. (1987). "Endocrine and metabolic effects of simple hysterectomy". International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 25 (6): 459–63. doi:10.1016/0020-7292(87)90062-2. PMID 2892704.
- ↑ Jassal SK, Barrett-Connor E, Edelstein SL (1995). "Low bioavailable testosterone levels predict future height loss in postmenopausal women". J. Bone Miner. Res. 10 (4): 650–4. doi:10.1002/jbmr.5650100419. PMID 7610937.
- ↑ Segraves R, Woodard T (2006). "Female hypoactive sexual desire disorder: History and current status". The journal of sexual medicine 3 (3): 408–18. doi:10.1111/j.1743-6109.2006.00246.x. PMID 16681466.
- ↑ Rivera, C. M.; Grossardt, B. R.; Rhodes, D. J.; Rocca, W. A. (2009). "Increased Mortality for Neurological and Mental Diseases following Early Bilateral Oophorectomy". Neuroepidemiology 33 (1): 32–40. doi:10.1159/000211951. PMC 2697609. PMID 19365140.
- ↑ Brown, J. S.; Sawaya, G.; Thom, D. H.; Grady, D. (2000). "Hysterectomy and urinary incontinence: a systematic review". The Lancet 356 (9229): 535–539. doi:10.1016/S0140-6736(00)02577-0. PMID 10950229.
- ↑ Altman, D.; Granath, F.; Cnattingius, S.; Falconer, C. (2007). "Hysterectomy and risk of stress-urinary-incontinence surgery: nationwide cohort study". The Lancet 370 (9597): 1494–1499. doi:10.1016/S0140-6736(07)61635-3. PMID 17964350.
- ↑ McPherson K, Herbert A, Judge A, et al. (2005). "Self-reported bladder function five years post-hysterectomy". Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology 25 (5): 469–75. doi:10.1080/01443610500235170. PMID 16183583.
- ↑ Lukanovic, A; Drazic, K (2010). "Risk factors for vaginal prolapse after hysterectomy". International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 110 (1): 27–30. doi:10.1016/j.ijgo.2010.01.025. PMID 20362288.
- ↑ Altman, D; Falconer, C; Cnattingius, S; Granath, F (2008). "Pelvic organ prolapse surgery following hysterectomy on benign indications". American Journal of Obstetrics and Gynecology 198 (5): 572.e1–572.e6. doi:10.1016/j.ajog.2008.01.012. PMID 18355787.
- ↑ Wiseman DM (2008). "Disorders of adhesions or adhesion-related disorder: monolithic entities or part of something bigger—CAPPS?". Semin. Reprod Med. 26 (4): 356–68. doi:10.1055/s-0028-1082394. PMID 18756413.
- ↑ Altman, D.; Yin, L.; Johansson, A.; Lundholm, C.; Grönberg, H. (2010). "Risk of Renal Cell Carcinoma After Hysterectomy". Archives of Internal Medicine 170 (22): 2011–2016. doi:10.1001/archinternmed.2010.425. PMID 21149759.
- ↑ Gago-Dominguez, M.; Castelao, J. E.; Yuan, J. M.; Ross, R. K.; Yu, M. C. (1999). "Increased risk of renal cell carcinoma subsequent to hysterectomy". Cancer Epidemiology, Biomarkers & Prevention 8 (11): 999–1003. PMID 10566555.
- ↑ Zucchetto, A.; Talamini, R.; Dal Maso, L.; Negri, E.; Polesel, J.; Ramazzotti, V.; Montella, M.; Canzonieri, V.; Serraino, D.; La Vecchia, C.; Franceschi, S. (2008). "Reproductive, menstrual, and other hormone-related factors and risk of renal cell cancer". International Journal of Cancer 123 (9): 2213–2216. doi:10.1002/ijc.23750. PMID 18711701.
- ↑ Cocks, P. S. (1980). "Early ectopic pregnancy after vaginal hysterectomy two case reports". BJOG: an International Journal of Obstetrics and Gynaecology 87 (5): 363. doi:10.1111/j.1471-0528.1980.tb04559.x.
- ↑ Milsom, I. (2007). "The levonorgestrel-releasing intrauterine system as an alternative to hysterectomy in peri-menopausal women". Contraception 75 (6): S152–S154. doi:10.1016/j.contraception.2007.01.003. PMID 17531608.
- ↑ Pesmen, Curt (July 27, 2007) 5 operations you don't want to get – and what to do instead. CNN
- ↑ Zapata, Lauren B.; Whiteman, Maura K.; Tepper, Naomi K.; Jamieson, Denise J.; Marchbanks, Polly A.; Curtis, Kathryn M. (2010). "Intrauterine device use among women with uterine fibroids: a systematic review☆". Contraception 82 (1): 41–55. doi:10.1016/j.contraception.2010.02.011. PMID 20682142.
- ↑ Parker, William H. and Parker, Rachel L. (2002) "A Gynecologist's Second Opinion: The Questions & Answers You Need to Take Charge of Your Health," Plume; Rev ed., 89–92, 105–150.
- ↑ Gupta JK, Sinha A, Lumsden MA, Hickey M (2012). "Uterine artery embolization for symptomatic uterine fibroids". In Gupta, Janesh K. Cochrane Database Syst Rev 5: CD005073. doi:10.1002/14651858.CD005073.pub3. PMID 22592701.
- ↑ Frederick R. Jelovsek, "Having Prolapse, Cystocele and Rectocele Fixed Without Hysterectomy"
- ↑ Hudson's FTM Resource Guide, "FTM Gender Reassignment Surgery, retrieved May 9, 2007.
- ↑ Hudson's FTM Resource Guide, "Types of Hysterectomy", retrieved May 8, 2007.
External links
- Hysterectomy on the Open Directory Project
- Medline article on Hysterectomy
- Oncolex.org features live footage videos showing radical hysterctomies
| |||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||