Hyperforin
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
| (1R,5S,6R,7S)-4-hydroxy-6-methyl-1,3,7-tris(3-methylbut-2-en-1-yl)-6-(4-methylpent-3-en-1-yl)-5-(2-methylpropanoyl)bicyclo[3.3.1]non-3-ene-2,9-dionemethylpropanoyl)bicyclo[3.3.1]non-3-ene-2,9-dione | |
| Clinical data | |
| Legal status | Unscheduled |
| Dependence liability | Nil |
| Routes | Oral |
| Pharmacokinetic data | |
| Metabolism | Hepatic and CYP3A & CYP2B |
| Half-life | 9-19.64 hours |
| Identifiers | |
| ATC code | None |
| PubChem | CID 114787 |
| DrugBank | DB01892 |
| ChemSpider | 16736597 |
| UNII | RM741E34FP |
| KEGG | C07608 |
| ChEBI | CHEBI:5834 |
| Chemical data | |
| Formula | C35H52O4 |
| Mol. mass | 536.784973 |
| SMILES
| |
| |
| Physical data | |
| Melt. point | 79-80 °C (-33 °F) |
| Solubility in water | 0.66 mg/mL (20 °C) |
| | |
Hyperforin is a phytochemical produced by some of the members of the plant genus Hypericum, notably Hypericum perforatum (St John's wort). It is believed to be one of the chief active constituents of St. John's wort (along with hypericin, pseudohypericin, adhyperforin and several flavonoid constituents).
Occurrence
Hyperforin has only been found in significant amounts in Hypericum perforatum (St. John's wort) with other related species such as Hypericum calycinum (Greater St. John's wort or Aaron's beard) containing lower levels of the phytochemical. It is thought to be a reuptake inhibitor.[1] It accumulates in oil glands, pistils, and fruits, probably as a plant defense against herbivory.[2] Other Hypericum species contain low amounts of hyperforin.[3]
Chemistry
Hyperforin is a prenylated phloroglucinol derivative. The structure of hyperforin was elucidated by a research group from the Shemyakin Institute of Bio-organic Chemistry (USSR Academy of Sciences in Moscow) and published in 1975.[4][5] A total synthesis of the non-natural enantiomer of hyperforin was reported in 2010[6] and a total synthesis of the natural enantiomer was disclosed in 2012.[7]
Hyperforin is unstable in the presence of light and oxygen.[8]
Pharmacokinetics
Some pharmacokinetic data on hyperforin is available for an extract containing 5% hyperforin. Maximal plasma levels (Cmax) in human volunteers were reached 3.5h after administration of an extract containing 14.8 mg hyperforin. Biological half-life (t1/2) and mean residence time were 9h and 12h respectively with an estimated steady state plasma concentration of 100 ng/mL (approx. 180 nM) for 3 doses/d. Linear plasma concentrations were observed within a normal dosage range and no accumulation occurred.[9]
In healthy male volunteers, 612 mg dry extract of St. John's wort produced hyperforin pharmacokinetics characterised by a half life of 19.64 hours.[10] It appears to be metabolised, at least in part, by CYP3A and CYP2B into hydroxyl metabolites.[11]
Biologic effects
Hyperforin is believed to be the primary active constituent responsible for the antidepressant and anxiolytic properties of the extracts of St. John's wort.[12] It acts as a reuptake inhibitor of monoamines, including serotonin, norepinephrine, dopamine, and of GABA and glutamate, with IC50 values of 0.05-0.10 μg/mL for all compounds, with the exception of glutamate, which is in the 0.5 μg/mL range.[13] Hyperforin also inhibits the reuptake of glycine[14] and choline (IC50=8.5μM).[15] It also modulates acetylcholine release in rat hippocampus and facilitates acetylcholine release in the striatum.[16][17] It appears to exert these effects by activating the transient receptor potential ion channel TRPC6.[18] Activation of TRPC6 induces the entry of sodium and calcium into the cell which causes inhibition of monoamine reuptake.[18] It also antagonises the NMDA receptor, AMPA receptor and GABA receptors.[19][20]
Its action on TRPC6 may enable it to protect from ischaemic brain injury.[21] Its action on the TRPC6 cation channel is also believed to be responsible for its BDNF-like modulation of dendritic spine morphology in hippocampal pyramidal neurons.[22]
Hyperforin also induces cytochrome P450 enzymes CYP3A4 and CYP2C9 by binding to and activating the pregnane X receptor (PXR).[23] The induction of CYP3A caused by hypericum perforatum seems to be heavily dependent on its hyperforin content.[24]
Hyperforin has been found to be a potent inhibitor of COX-1 and 5-LO with IC50 values of 300nM and 90nM respectively, giving it an anti-inflammatory action of approximately 3-18 times stronger than aspirin.[25]
Hyperforin has topical antibiotic properties and is active against methicillin-resistant strains of Staphylococcus aureus (MRSA) with a minimal inhibitory concentration (MIC) value of 1.0 μg/mL (1.86μM),[26] as well as against other gram-positive bacteria.[27] Chemical analogues of hyperforin have also exhibited in vitro activity against various bacterial species.[28] Hyperforin also has in vitro antimalarial effects.[29]
Procognitive effects of hyperforin have been observed in rats.[11] In vivo evidence suggests its efficacy against Alzheimer's disease,[19][30] an action it seems to share with its analogue, tetrahydrohyperforin.[31][32][33]
Hyperforin also has anticancer effects, both in vitro and in vivo, which are likely the result of its both anti-angiogenic and pro-apoptotic effects.[34] Several chemical analogues of hyperforin have also exhibited anticancer effects in vitro and in vivo.[35][36][37] Hyperforin also has anticlastogenic effects.[38] Hyperforin also inhibits SIRT1 and SIRT2 with a IC50 of 15±0.5μM and 28±0.2μM respectively.[39]
In 2008 it was reported that men with premature ejaculation that were administered an immediate-release oral formulation reported lasting longer.[40] Hyperforin promotes mitochondrial function and the development of oligodendrocytes.[41]
Extracts of hypericum perforatum and the hyperforin, analogue, IDN-5491 (hyperforin-trimethoxybenzoate) possess antidepressant-like effects that are dependent on the sigma and D2 receptors.[42]
| Hyperforin and its semi-synthetic analogues | |||
 Hyperforin |  Aristoforin |  Hyperforin trimethoxybenzoate |  Tetrahydrohyperforin |
 Octahydrohyperforin | 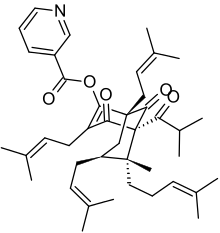 Hyperforin nicotinate | ||
Reuptake Inhibition
| Neurotransmitter | IC50 (nM) |
|---|---|
| Norepinephrine | 80 ± 24 |
| Dopamine | 102 ± 19 |
| GABA | 184 ± 41 |
| 5-HT | 205 ± 45 |
| L-Glutamate | 829 ± 687 |
| Choline | 8500 |
See also
- St John's Wort
- Serotonin
- Hypericin
- Adhyperforin
- Hypericum
- Reuptake inhibitor
Further reading
- Beerhues L (October 2006). "Molecule of Interest: Hyperforin". Phytochemistry 67 (20): 2201–7. doi:10.1016/j.phytochem.2006.08.017. PMID 16973193.
- http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?q=all&cid=441298#ec
References
- ↑ Roz N, Rehavi M. (Jun 2003). "Hyperforin inhibits vesicular uptake of monoamines by dissipating pH gradient across synaptic vesicle membrane.". Life Sciences 73 (4): 461–70. PMID 12759140.
- ↑ Beerhues L. (2006). "Hyperforin." Phytochemistry 67 (20): 2201-7. PMID 16973193
- ↑ Smelcerovic A, Spiteller M (March 2006). "Phytochemical analysis of nine Hypericum L. species from Serbia and the F.Y.R. Macedonia". Die Pharmazie 61 (3): 251–2. PMID 16599273.
- ↑ Bystrov NS, Gupta ShR, Dobrynin VN, Kolosov MN, Chernov BK (January 1976). "[Structure of the antibiotic hyperforin]". Doklady Akademii Nauk SSSR (in Russian) 226 (1): 88–90. PMID 1248360.
- ↑ Bystrov NS, Chernov BK, Dobrynin VN, Kolosov MN (1975). "[The structure of hyperforin]". Tetrahedron Letters 16 (32): 2791–2794. doi:10.1016/S0040-4039(00)75241-5.
- ↑ Shimizu Y, Shi S-L, Usuda H, Kanai M, Shibasaki M (February 2010). "Catalytic Asymmetric Total Synthesis of ent-Hyperforin". Angew Chem Int Ed 49 (6): 1103–6. doi:10.1002/anie.200906678.
- ↑ Sparling B, Moebius D, Shair M (December 2012). "Enantioselective Total Synthesis of Hyperforin". J Am Chem Soc 135 (2): 644–7. doi:10.1021/ja312150d. PMID 23270309.
- ↑ Liu, F; Pan, C; Drumm, P; Ang, CY (February 2005). "Liquid chromatography-mass spectrometry studies of St. John's wort methanol extraction: active constituents and their transformation". Journal of pharmaceutical and biomedical analysis 37 (2): 303–12. doi:10.1016/j.jpba.2004.10.034. PMID 15708671.
- ↑ Biber, A; Fischer, H; Römer, A; Chatterjee, SS (June 1998). "Oral bioavailability of hyperforin from hypericum extracts in rats and human volunteers". Pharmacopsychiatry 31 (Suppl 1): 36–43. doi:10.1055/s-2007-979344. PMID 9684946.
- ↑ Schulz, HU; Schürer, M; Bässler, D; Weiser, D (2005). "Investigation of the Bioavailability of Hypericin, Pseudohypericin, Hyperforin and the Flavonoids Quercetin and Isorhamnetin Following Single and Multiple Oral Dosing of a Hypericum Extract Containing Tablet". Arzneimittelforschung 55 (1): 15–22. doi:10.1055/s-0031-1296820. PMID 15727160.
- ↑ 11.0 11.1 Zanoli, P (Fall 2004). "Role of hyperforin in the pharmacological activities of St. John's Wort" (PDF). CNS Drugs Reviews 10 (3): 203–218. doi:10.1111/j.1527-3458.2004.tb00022.x. PMID 15492771.
- ↑ Newall, Carol A.; Joanne Barnes; Anderson, Linda R. (2002). Herbal medicines: a guide for healthcare professionals. London: Pharmaceutical Press. ISBN 0-85369-474-5.
- ↑ Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Müller WE (1998). "Hyperforin as a possible antidepressant component of hypericum extracts". Life Sci. 63 (6): 499–510. doi:10.1016/S0024-3205(98)00299-9. PMID 9718074.
- ↑ Marsh WL, Davies JA (October 2002). "The involvement of sodium and calcium ions in the release of amino acid neurotransmitters from mouse cortical slices elicited by hyperforin". Life Sciences 71 (22): 2645–55. doi:10.1016/S0024-3205(02)02104-5. PMID 12354583.
- ↑ Buchholzer ML, Dvorak C, Chatterjee SS, Klein J (May 2002). "Dual modulation of striatal acetylcholine release by hyperforin, a constituent of St. John's wort". The Journal of Pharmacology and Experimental Therapeutics 301 (2): 714–9. doi:10.1124/jpet.301.2.714. PMID 11961077.
- ↑ Buchholzer, ML; Dvorak, C; Chatterjee, SS; Klein, J (May 2002). "Dual modulation of striatal acetylcholine release by hyperforin, a constituent of St. John's wort" (PDF). The Journal of Pharmacology and Experimental Therapeutics 301 (2): 714–719. doi:10.1124/jpet.301.2.714. PMID 11961077.
- ↑ Kiewert, C; Buchholzer, ML; Hartmann, J; Chatterjee, SS; Klein, J (July 2004). "Stimulation of hippocampal acetylcholine release by hyperforin, a constituent of St. John's Wort". Neuroscience Letters 364 (3): 195–198. doi:10.1016/j.neulet.2004.04.046. PMID 15196674.
- ↑ 18.0 18.1 Leuner K, Kazanski V, Müller M, et al. (December 2007). "Hyperforin--a key constituent of St. John's wort specifically activates TRPC6 channels". The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 21 (14): 4101–11. doi:10.1096/fj.07-8110com. PMID 17666455.
- ↑ 19.0 19.1 Griffith, TN; Varela-Nallar, L; Dinamarca, MC; Inestrosa, NC (2010). "Neurobiological effects of Hyperforin and its potential in Alzheimer's disease therapy". Current Medicinal Chemistry 17 (5): 391–406. doi:10.2174/092986710790226156. PMID 20015041.
- ↑ Medina, MA; Martínez-Poveda, B; Amores-Sánchez, MI; Quesada, AR (June 2006). "Hyperforin: More than an antidepressant bioactive compound?". Life Sciences 79 (2): 105–111. doi:10.1016/j.lfs.2005.12.027.
- ↑ Lin, Y; Zhang, JC; Fu, J; Chen, F; Wang, J; Wu, ZL; Yuan, SY (February 2013). "Hyperforin attenuates brain damage induced by transient middle cerebral artery occlusion (MCAO) in rats via inhibition of TRPC6 channels degradation". Journal of Cerebral Blood Flow and Metabolism 33 (2): 253–262. doi:10.1038/jcbfm.2012.164. PMID 23149561.
- ↑ Leuner, K; Li, W; Amaral, MD; Rudolph, S; Calfa, G; Schuwald, AM; Harteneck, C; Inoue, T; Pozzo-Miller, L. "Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca(2+) -permeable TRPC6 channels". Hippocampus 23 (1): 40–52. doi:10.1002/hipo.22052. PMC 3538039. PMID 22815087.
- ↑ Moore LB, Goodwin B, Jones SA, et al. (June 2000). "St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor". Proceedings of the National Academy of Sciences of the United States of America 97 (13): 7500–2. doi:10.1073/pnas.130155097. PMC 16574. PMID 10852961.
- ↑ Mueller, SC; Majcher-Peszynska, J; Mundkowski, RG; Uehleke, B; Klammt, S; Sievers, H; Lehnfeld, R; Frank, B; Thurow, K; Kundt, G; Drewelow, B (January 2009). "No clinically relevant CYP3A induction after St. John’s wort with low hyperforin content in healthy volunteers". European Journal of Clinical Pharmacology 65 (1): 81–87. doi:10.1007/s00228-008-0554-y. PMID 18762932.
- ↑ Albert, D; Zündorf, I; Dingermann, T; Müller, WE; Steinhilber, D; Werz, O (December 2002). "Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase". Biochemical Pharmacology 64 (12): 1767–1775. doi:10.1016/S0006-2952(02)01387-4. PMID 12445866.
- ↑ Reichling, J; Weseler, A; Saller, R (July 2001). "A current review of the antimicrobial activity of Hypericum perforatum L". Pharmacopsychiatry. 34 Suppl 1: S116–8. doi:10.1055/s-2001-15514. PMID 11518059.
- ↑ Schempp, CM; Pelz, K; Wittmer, A; Schöpf, E; Simon, JC (June 1999). "Antibacterial activity of hyperforin from St John's wort, against multiresistant Staphylococcus aureus and gram-positive bacteria". Lancet 353 (9170): 2129. doi:10.1016/S0140-6736(99)00214-7. PMID 10382704.
- ↑ Schiavone, BI; Rosato, A; Marilena, M; Gibbons, S; Bombardelli, E; Verotta, L; Franchini, C; Corbo, F (September 2013). Biological Evaluation of Hyperforin and Its Hydrogenated Analogue on Bacterial Growth and Biofilm Production 76 (9). pp. 1819–1823. doi:10.1021/np400394c. PMID 23981190.
- ↑ Verotta, L; Appendino, G; Bombardelli, E; Brun, R (March 2007). "In vitro antimalarial activity of hyperforin, a prenylated acylphloroglucinol. A structure–activity study". Bioorganic & Medicinal Chemistry Letters 17 (6): 1544–1548. doi:10.1016/j.bmcl.2006.12.100. PMID 17234416.
- ↑ Dinamarca, MC; Cerpa, W; Garrido, J; Hancke, JL; Inestrosa, NC (November 2006). "Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits" (PDF). Molecular Psychiatry 11 (11): 1032–1048. doi:10.1038/sj.mp.4001866. PMID 16880827.
- ↑ Carvajal, FJ; Zolezzi, JM; Tapia-Rojas, C; Godoy, JA; Inestrosa, NC (January 2013). "Tetrahydrohyperforin decreases cholinergic markers associated with amyloid-β plaques, 4-hydroxynonenal formation, and caspase-3 activation in AβPP/PS1 mice". Journal of Alzheimer's Disease 36 (1): 99–118. doi:10.3233/JAD-130230. PMID 23568104.
- ↑ Cerpa, W; Hancke, JL; Morazzoni, P; Bombardelli, E; Riva, A; Marin, PP; Inestrosa, NC (March 2010). "The hyperforin derivative IDN5706 occludes spatial memory impairments and neuropathological changes in a double transgenic Alzheimer's mouse model". Current Alzheimer Research 7 (2): 126–133. doi:10.2174/156720510790691218. PMID 19939230.
- ↑ Carvajal, FJ; Inestrosa, NC (September 2011). "Interactions of AChE with Aβ Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706" (PDF). Frontiers in Molecular Neuroscience 4: 19. doi:10.3389/fnmol.2011.00019. PMC 3172730. PMID 21949501.
- ↑ Quiney, C; Billard, C; Salanoubat, C; Fourneron, JD; Kolb, JP (September 2006). "Hyperforin, a new lead compound against the progression of cancer and leukemia?" (PDF). Leukemia 20 (9): 1519–1525. doi:10.1038/sj.leu.2404301. PMID 16791262.
- ↑ Martínez-Poveda, B; Verotta, L; Bombardelli, E; Quesada, AR; Medina, MA (March 2010). "Tetrahydrohyperforin and Octahydrohyperforin Are Two New Potent Inhibitors of Angiogenesis" (PDF). PLoS ONE 5 (3): e9558. doi:10.1371/journal.pone.0009558. PMC 2835552. PMID 20224821.
- ↑ Sun, F; Liu, JY; He, F; Liu, Z; Wang, R; Wang, DM; Wang, YF; Yang, DP (August 2011). "In-vitro antitumor activity evaluation of hyperforin derivatives". Journal of Asian Natural Products Research 13 (8): 688–699. doi:10.1080/10286020.2011.584532. PMID 21751836.
- ↑ Gartner, M; Müller, T; Simon, JC; Giannis, A; Sleeman, JP (January 2005). "Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent". Chembiochem 6 (1): 171–177. doi:10.1002/cbic.200400195. PMID 15593112.
- ↑ Imreova, P; Miadokova, C; Galova, E; Chankova, S; Chalupa, I. "POTENTIAL ANTICLASTOGENIC EFFECT OF HYPERFORIN" (PDF). Military Medical Science Letters 82: 1–5. ISSN 0372-7025.
- ↑ Gey, C; Kyrylenko, S; Hennig, L; Nguyen, LH; Büttner, A; Pham, HD; Giannis, A. "Phloroglucinol Derivatives Guttiferone G, Aristoforin, and Hyperforin: Inhibitors of Human Sirtuins SIRT1 and SIRT2". Angewandte Chemie International Edition 46 (27): 5219–5222. doi:10.1002/anie.200605207. PMID 17516596.
- ↑ Kim, DK; Chancellor, MB (May–June 2008). "Men reporting lasting longer with hyperforin" (PDF). International Braz J Urol 34 (3): 370–371. doi:10.1590/S1677-55382008000300017. PMID 18601770.
- ↑ Wang, Y; Zhang, Y; He, J; Zhang, H; Xiao, L; Nazarali, A; Zhang, Z; Zhang, D; Tan, Q; Kong, J; Li, XM (November 2011). "Hyperforin promotes mitochondrial function and development of oligodendrocytes" (PDF). Journal of Neurochemistry 119 (3): 555–568. doi:10.1111/j.1471-4159.2011.07433.x. PMID 21848657.
- ↑ Cervo, L; Mennini, T; Rozio, M; Ekalle-Soppo, CB; Canetta, A; Burbassi, S; Guiso, G; Pirona, L; Riva, A; Morazzoni, P; Caccia, S; Gobbi, M (March 2005). "Potential antidepressant properties of IDN 5491 (hyperforin-trimethoxybenzoate), a semisynthetic ester of hyperforin". European Neuropsychopharmacology 15 (2): 211–218. doi:10.1016/j.euroneuro.2004.07.004. PMID 15695067.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||