Hydrophilic-lipophilic balance
The Hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic, determined by calculating values for the different regions of the molecule, as described by Griffin in 1949[1] and 1954.[2] Other methods have been suggested, notably in 1957 by Davies.[3]
Griffin's method
Griffin's method for non-ionic surfactants as described in 1954 works as follows:
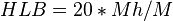
where Mh is the molecular mass of the hydrophilic portion of the molecule, and M is the molecular mass of the whole molecule, giving a result on a scale of 0 to 20. An HLB value of 0 corresponds to a completely lipophilic/hydrophobic molecule, and a value of 20 corresponds to a completely hydrophilic/lipophobic molecule.
The HLB value can be used to predict the surfactant properties of a molecule:
- < 10 : Lipid soluble (water insoluble)
- > 10 : Water soluble (lipid insoluble)
- 4 to 8: anti-foaming agent
- 7 to 11: W/O (water in oil) emulsifier
- 11 to 14: wetting agent
- 12 to 15: detergent
- 12 to 16: O/W (oil in water) emulsifier
- 16 to 20: solubiliser or hydrotrope
Davies' method
In 1957, Davies suggested a method based on calculating a value based on the chemical groups of the molecule. The advantage of this method is that it takes into account the effect of stronger and weaker hydrophilic groups. The method works as follows:

with:
m - Number of hydrophilic groups in the molecule
Hh - Value of the hydrophilic groups
n - Number of lipophilic groups in the molecule
Hl - Value of the lipophilic groups
References
- ↑ Griffin, William C. (1949), "Classification of Surface-Active Agents by 'HLB'", Journal of the Society of Cosmetic Chemists 1 (5): 311-26
- ↑ Griffin, William C. (1954), "Calculation of HLB Values of Non-Ionic Surfactants", Journal of the Society of Cosmetic Chemists 5 (4): 249-56
- ↑ Davies JT (1957), "A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent", Gas/Liquid and Liquid/Liquid Interface (Proceedings of the International Congress of Surface Activity): 426–38