Hydrohalogenation
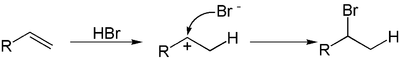
A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.[1][2][3]
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents, an observation known as Markovnikov's rule. This is due to the abstraction of a hydrogen atom by the alkene from the acid (HX) to form the most stable carbocation(relative stability: 3°>2°>1°>methyl), as well as generating a halogen anion.
The subsequent reaction proceeds by an SN1 mechanism due to the presence of the electrophilic carbocation and a nucleophilic halide anion, thus resulting in the final product.
A simple example of a hydrochlorination is that of indene with hydrochloric acid gas (no solvent):[4]
Anti-Markovnikov addition
In the presence of peroxides, HBr adds to a given alkene in an anti-Markovnikov addition fashion.[5] This is because the reaction proceeds through the most stable carbon radical intermediate (relative stability: 3° > 2° > 1°>methyl) instead of a carbocation. The mechanism for this reaction is similar to a chain reaction such as free radical halogenation in which the peroxide promotes the formation of the bromide radical. Therefore, in the presence of peroxides, HBr adds so that the bromine atom is added to the carbon bearing the most numerous hydrogen substituents and hydrogen atoms will add to carbons bearing less hydrogen substituents. However, this process is restricted to addition of HBr.
No other hydrogen halide behaves in the manner described above, this can be explained by a survey of the different hydrohalic acids: HF (hydrogen fluoride), HCl (hydrogen chloride — more commonly known by the aqueous species hydrochloric acid), and HI (hydrogen iodide).
The hydrogen-fluorine bond is simply too strong and therefore no fluorine radicals can be generated in the propagation step. Hydrogen chloride will react in a manner that is so slow that it is essentially synthetically useless. This is because the hydrogen-chlorine bond is strong and thus the second step of the reaction would be extremely slow due to the heat required (it is an endothermic reaction). Due to the weakness of the carbon-iodine bond necessary to complete the first step of the propagation phase, insufficient heat is released to proceed through the reaction successfully.
With Michael acceptors the addition is also anti-Markovnikov because now a nucleophilic X- reacts in a nucleophilic conjugate addition for example in the reaction of HCl with acrolein.[6]
Scope
Recent research has found that adding silica gel or alumina to H-Cl (or H-Br) in dichloromethane increases the rate of reaction making it an easy one to carry out [citation needed].
References
- ↑ Solomons, T.W. Graham; Fryhle, Craig B. (2003), Organic Chemistry (8th ed.), Wiley, ISBN 0-471-41799-8
- ↑ Smith, Janice G. (2007), Organic Chemistry (2nd ed.), McGraw-Hill, ISBN 0-07-332749-2
- ↑ P.J. Kropp, K.A. Dans, S.D. Crawford, M.W. Tubergen, K.D. Kepler, S.L Craig, and V.P. Wilson (1990), "Surface-mediated reactions. 1. Hydrohalogenation of alkenes and alkynes", J. Am. Chem. Soc. 112 (112): 7433–7434, doi:10.1021/ja00176a075.
- ↑ R. A. Pacaud and C. F. H. Allen, "α-Hydroindone", Org. Synth.; Coll. Vol. 2: 336
- ↑ March Jerry; (1885). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- ↑ C. Moureu and R. Chaux (1941), "β-Chloropropionic acid", Org. Synth.; Coll. Vol. 1: 166