Herbacetin
From Wikipedia, the free encyclopedia
| Herbacetin | |
|---|---|
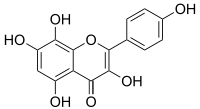 | |
| IUPAC name 3,5,7,8-tetrahydroxy-2-(4-hydroxyphenyl)chromen-4-one | |
| Other names 8-Hydroxykaempferol | |
| Identifiers | |
| CAS number | 527-95-7 |
| PubChem | 5280544 |
| ChemSpider | 4444174 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C15H10O7 |
| Molar mass | 302.24 g mol−1 |
| Density | 1.799 g/mL |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Herbacetin is a flavonol, a type of flavonoid.
Glycosides
Herbacetin diglucoside can be isolated from flaxseed hulls.[1]
Rhodionin is a herbacetin rhamnoside found in Rhodiola species.[2]
Other related compounds
Rhodiolin, a flavonolignan, is the product of the oxidative coupling of coniferyl alcohol with the 7,8-dihydroxy grouping of herbacetin. It can be found in the rhizome of Rhodiola rosea.[3]
References
- ↑ Struijs, K.; Vincken, J. P.; Verhoef, R.; Van Oostveen-Van Casteren, W. H. M.; Voragen, A. G. J.; Gruppen, H. (2007). "The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls". Phytochemistry 68 (8): 1227–1235. doi:10.1016/j.phytochem.2006.10.022. PMID 17141814.
- ↑ Li, T.; Zhang, H. (2008). "Identification and Comparative Determination of Rhodionin in Traditional Tibetan Medicinal Plants of Fourteen Rhodiola Species by High-Performance Liquid Chromatography-Photodiode Array Detection and Electrospray Ionization-Mass Spectrometry". Chemical & Pharmaceutical Bulletin 56 (6): 807–14. doi:10.1248/cpb.56.807. PMID 18520085.
- ↑ Zapesochnaya, G. G.; Kurkin, V. A. (1983). "The flavonoids of the rhizomes ofRhodiola rosea. II. A flavonolignan and glycosides of herbacetin". Chemistry of Natural Compounds 19: 21. doi:10.1007/BF00579955.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.