Hepatocyte
| Hepatocite | |
|---|---|
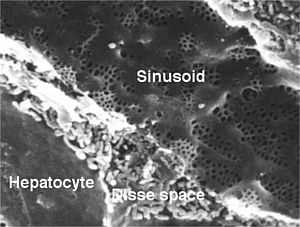 | |
| Hepatocyte and sinusoid (blood vessel) in a rat liver with fenestrated endothelial cells. Fenestration are approx 100 nm diameter, and the sinusoidal width 5 µm. | |
 | |
| Cross-section of the human liver. | |
| Latin | Hepatocytus |
| Code | TH H3.04.05.0.00006 |
A hepatocyte is a cell of the main tissue of the liver. Hepatocytes make up 70-85% of the liver's cytoplasmic mass. These cells are involved in:
- Protein synthesis
- Protein storage
- Transformation of carbohydrates
- Synthesis of cholesterol, bile salts and phospholipids
- Detoxification, modification, and excretion of exogenous and endogenous substances
The hepatocyte also initiates formation and secretion of bile.
Structure
The typical hepatocyte forms a cubical cell of 15 µm sides (in comparison, a human hair has a diameter of 17 to 180 µm). The typical volume of a hepatocyte is 3.4 x 10−9 cm3.[1]
The smooth endoplasmic reticulum (Smooth ER) is an abundant organelle in hepatocytes, whereas most cells in the body have small amounts of smooth ER.[2]
Histology
Hepatocytes display an eosinophilic cytoplasm, reflecting numerous mitochondria, and basophilic stippling due to large amounts of rough endoplasmic reticulum and free ribosomes. Brown lipofuscin granules are also observed (with increasing age) together with irregular unstained areas of cytoplasm; these correspond to cytoplasmic glycogen and lipid stores removed during histological preparation. The average life span of the hepatocyte is 5 months; they are able to regenerate.
Hepatocyte nuclei are round with dispersed chromatin and prominent nucleoli. Anisokaryosis is common and often reflects tetraploidy and other degrees of polyploidy, a normal feature of 30-40% of hepatocytes in adult human liver.[3] Binucleate cells are also common.
Hepatocytes are organised into plates separated by vascular channels (sinusoids), an arrangement supported by a reticulin (collagen type III) network. The hepatocyte plates are one cell thick in mammals and two cells thick in the chicken. Sinusoids display a discontinuous, fenestrated endothelial cell lining. The endothelial cells have no basement membrane and are separated from the hepatocytes by the space of Disse, which drains lymph into the portal tract lymphatics.
Kupffer cells are scattered between endothelial cells; they are part of the reticuloendothelial system and phagocytose spent erythrocytes. Stellate (Ito) cells store vitamin A and produce extracellular matrix and collagen; they are also distributed amongst endothelial cells but are difficult to visualise by light microscopy.
Hepatocytes are an important physiological example for evalutation of both biological and metabolic effects of xenobiotics. They are separated from the liver by collagenase digestion, which is a two step process. In the first step, the liver is placed in an isotonic solution, in which calcium is removed to disrupt cell-cell tight junctions by the use of a calcium chelating agent. Next, a solution containing collagenase is added to separate the hepatocytes from the liver stroma. This process creates a suspension of hepatocytes, which can be cultured and plated on 96 well plates for immediate use, or cryopreserved by freezing.[4] They do not proliferate in culture. Hepatocytes are intensely sensitive to damage during the cycles of cryopreservation including freezing and thawing. Even after the addition of classical cryoprotectants there is still damage done while being cryopreserved.[5]
Protein synthesis
The hepatocyte is a cell in the body that manufactures serum albumin, fibrinogen, and the prothrombin group of clotting factors (except for Factors 3 and 4).
It is the main site for the synthesis of lipoproteins, ceruloplasmin, transferrin, complement, and glycoproteins. Hepatocytes manufacture their own structural proteins and intracellular enzymes.
Synthesis of proteins is by the rough endoplasmic reticulum (RER), and both the rough and smooth endoplasmic reticulum (SER) are involved in secretion of the proteins formed.
The endoplasmic reticulum (ER) is involved in conjugation of proteins to lipid and carbohydrate moieties synthesized by, or modified within, the hepatocytes.
Carbohydrate metabolism
The liver forms fatty acids from carbohydrates and synthesizes triglycerides from fatty acids and glycerol. Hepatocytes also synthesize apoproteins with which they then assemble and export lipoproteins (VLDL, HDL).
The liver is also the main site in the body for gluconeogenesis, the formation of carbohydrates from precursors such as alanine, glycerol, and oxaloacetate.
Lipid metabolism
The liver receives many lipids from the systemic circulation and metabolizes chylomicron remnants. It also synthesizes cholesterol from acetate and further synthesizes bile salts. The liver is the sole site of bile salts formation.
Detoxification
Hepatocytes have the ability to metabolize, detoxify, and inactivate exogenous compounds such as drugs, (drug metabolism), and insecticides, and endogenous compounds such as steroids.
The drainage of the intestinal venous blood into the liver requires efficient detoxification of miscellaneous absorbed substances to maintain homeostasis and protect the body against ingested toxins.
One of the detoxifying functions of hepatocytes is to modify ammonia into urea for excretion.
The most abundant organelle in liver cell is the smooth endoplasmic reticulum.
Additional images
-
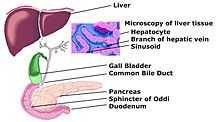
Schemic diagram of Biliary system
References
- ↑ Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D., Darnell, J. E. Molecular Cell Biology (Fifth Edition). W. H. Freeman and Company. New York, 2000, pp 10.
- ↑ Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D., Darnell, J. E. Molecular Cell Biology (Fifth Edition). W. H. Freeman and Company. New York, 2000, pp 168.
- ↑ Séverine Celton-Morizur; Grégory Merlen, Dominique Couton, Chantal Desdouets (February 2010). "Polyploidy and liver proliferation". Cell Cycle 9 (3). pp. 460–466. Retrieved 2 November 2011.
- ↑ Li, Albert P.; "Screening for human ADME/Tox drug properties in Drug Discovery": Drug Discovery Today, Vol. 6, No. 7, April 2001, pp. 357-366
- ↑ Hamel, F.; Grondin, M. L.; Denizeau, F.; Averill-Bates, D. A.; Sarhan, F. (2006). "Wheat extracts as an efficient cryoprotective agent for primary cultures of rat hepatocytes". Biotechnology and Bioengineering 95 (4): 661–670. doi:10.1002/bit.20953. PMID 16927246.
External links
- BU Histology Learning System: 22101ooa - "Ultrastructure of the Cell: hepatocytes and sinusoids"
- Hepatic Histology: Hepatocytes (Colorado State University
- The use of hepatocytes to measure clearance The performance of in vitro hepatic clearance studies including analysis of the data.
| |||||||||||||||||||||