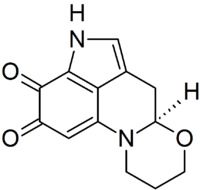
Haematopodin
| Haematopodin | ||
|---|---|---|
 | ||
| Identifiers | ||
| ChemSpider | 10473906 | |
| Jmol-3D images | {{#if:O=C3/C=C2/N4CCCO[C@@H]4Cc1cnc(c12)C3=O|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C13H12N2O3 | |
| Molar mass | 244.25 g mol−1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Haematopodin is the more stable breakdown product of Haematopodin B.[1] Both compounds are found in the mushroom Mycena haematopus, although haematopodin only occurs in trace amounts in fresh fruit bodies. Similar pigments (with the 1,3,4,5-tetrahydropyrrolo[4,3,2-de]quinoline structure), known as batzellins and damirones, have been found in sea sponges.[1] A chemical synthesis for haematopodin was reported in 1996. Key steps in the synthesis involved the addition of 3-[(2,4-dimethoxybenzyl)amino]-1-propanol to the indolo-6,7-quinone and cyclization of the resulting adduct with trifluoroacetic acid.[2]
References
- ↑ 1.0 1.1 Baumann, C.; Bröckelmann, M.; Fugmann, B; Steglich, W.; Sheldrick, W. S. (1993). "Pigments of fungi. 62. Haematopodin, an unusual pyrrologuinone derivative isolated from the fungus Mycena haematopus, Agaricales". Angewandte Chemie—International Edition in English 32 (7): 1087–89. doi:10.1002/anie.199310871.
- ↑ Hopmann, C.; Steglish, W. (1996). "Synthesis of haematopodin – A pigment from the mushroom Mycena haematopus (Basidiomycetes)". Liebigs Annalen 1996 (7): 1117–20. doi:10.1002/jlac.15719960710.