HU-345
From Wikipedia, the free encyclopedia
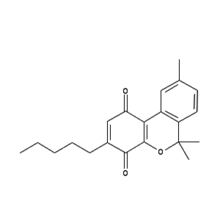 | |
|---|---|
| Systematic (IUPAC) name | |
| 6,6,9-trimethyl-3-pentyl-1H-benzo[c]chromene-1,4(6H)-dione | |
| Clinical data | |
| Legal status | ? |
| Identifiers | |
| ATC code | ? |
| PubChem | CID 11198119 |
| ChemSpider | 9373188 |
| ChEMBL | CHEMBL127671 |
| Chemical data | |
| Formula | C21H24O3 |
| Mol. mass | 324.414 |
| SMILES
| |
| |
| | |
HU-345 (cannabinol quinone) is a drug that is able to inhibit aortic ring angiogenesis more potently than its parent compound cannabinol.[1][2]
See also
References
- ↑ Natalya M. Kogan, et al. (2006). "A Cannabinoid Quinone Inhibits Angiogenesis by Targeting Vascular Endothelial Cells". Molecular Pharmacology 70 (1): 51–59. doi:10.1124/mol.105.021089. PMID 16571653.
- ↑ US patent 0092584, Mechoulam R, Kogan NM, Rabinowitz R, Schlesinger M, "Therapeutic Use of Quinonoid Derivatives of Cannabinoids", granted 2011-04-21
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.