Grubbs' catalyst
| 1st Generation Grubbs Catalyst | ||
|---|---|---|
 | ||
 | ||
| IUPAC name Benzylidene-bis(tricyclohexylphosphine)-dichlororuthenium | ||
| Identifiers | ||
| CAS number | 172222-30-9 | |
| PubChem | 24881009 | |
| Properties | ||
| Molecular formula | C43H72Cl2P2Ru | |
| Molar mass | 822.96 g mol−1 | |
| Appearance | Purple solid | |
| Melting point | 153 °C (decomposion) | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
| 2nd Generation Grubbs Catalyst | ||
|---|---|---|
 | ||
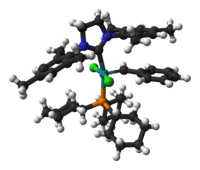 | ||
| IUPAC name [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium | ||
| Identifiers | ||
| CAS number | 246047-72-3 | |
| Properties | ||
| Molecular formula | C46H65Cl2N2PRu | |
| Molar mass | 848.97 g mol−1 | |
| Appearance | Pinkish brown solid | |
| Melting point | 143.5-148.5 °C | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
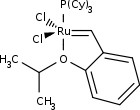
| 1st Generation Hoveyda-Grubbs Catalyst | ||
|---|---|---|
 | ||
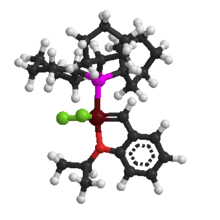 | ||
| IUPAC name Dichloro(o-isopropoxyphenylmethylene)(tricyclohexylphosphine)ruthenium(II) | ||
| Identifiers | ||
| CAS number | 203714-71-0 | |
| PubChem | 24880901 | |
| Properties | ||
| Molecular formula | C28H45Cl2OPRu | |
| Molar mass | 600.61 g mol−1 | |
| Appearance | Brown solid | |
| Melting point | 195-197 °C | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
| 2nd Generation Hoveyda-Grubbs Catalyst | ||
|---|---|---|
 | ||
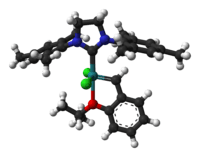 | ||
| IUPAC name [1,3-Bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(oisopropoxyphenylmethylene)ruthenium | ||
| Identifiers | ||
| CAS number | 301224-40-8 | |
| Properties | ||
| Molecular formula | C31H38Cl2N2ORu | |
| Molar mass | 626.62 g mol−1 | |
| Appearance | Green solid | |
| Melting point | 216-220 °C | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Grubbs' Catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who first synthesized them. There are two generations of the catalyst, as shown on the right.[1][2] In contrast to other olefin metathesis catalysts, Grubbs' catalysts tolerate other functional groups in the alkene, are air-tolerant and are compatible with a wide range of solvents.[3][4] For these reasons, Grubbs' catalysts have become popular in synthetic organic chemistry.[5]
First generation catalyst
The first well-defined ruthenium catalyst for olefin metathesis was discovered in 1992.[6] It was prepared from RuCl2(PPh3)4 and diphenylcyclopropene.
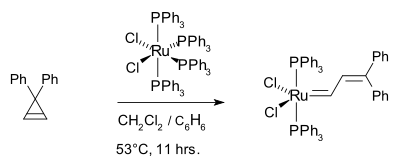
This initial ruthenium catalyst was followed in 1995 by what is now known as the first generation Grubbs catalyst. It is easily synthesized from RuCl2(PPh3)3, phenyldiazomethane, and tricyclohexylphosphine in a one-pot synthesis.[7][8]
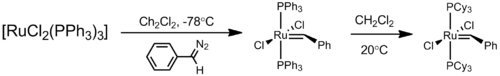
The first generation Grubbs catalyst, while largely replaced by the second generation catalyst in usage, was not only the first catalyst to be developed other than those developed by Richard R. Schrock (Schrock carbenes), but is also important as a precursor to all other Grubbs-type catalysts.
Second generation catalyst
The second generation catalyst has the same uses in organic synthesis as the first generation catalyst, but generally with higher activity. This catalyst is stable toward moisture and air, thus is easier to handle in the lab.
Shortly before the discovery of the 2nd generation Grubbs' catalyst, a very similar catalyst based on an unsaturated N-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)imidazole) was reported independently by Nolan[9] and Grubbs[10] in March 1999, and by Fürstner[11] in June of the same year. Shortly thereafter, in August 1999, Grubbs reported the 2nd generation catalyst, based on a saturated N-heterocyclic carbene (1,3-bis(2,4,6-trimethylphenyl)dihydroimidazole):[12]
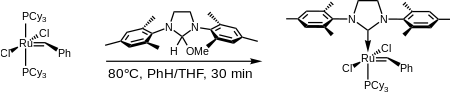
In both the saturated and unsaturated cases a phosphine ligand is replaced with an N-heterocyclic carbene (NHC), which is characteristic of all 2nd generation type catalysts.[3]
Both the 1st and 2nd generation catalysts are commercially available, along with many derivatives of the 2nd generation catalyst.
Hoveyda–Grubbs Catalyst
In the Hoveyda–Grubbs Catalysts, the benzylidene ligands have a chelating ortho-isopropoxy group attached to the benzene rings. The ortho-isopropoxybenzylidene moiety is sometimes referred to as a Hoveyda chelate. The chelating oxygen atom replaces a phosphine ligand, which in the case of the 2nd generation catalyst, gives a completely phosphine-free structure. The 1st generation Hoveyda–Grubbs catalyst was reported in 1999 by the Hoveyda group,[13] and in the following year, the 2nd generation Hoveyda–Grubbs catalyst was described in nearly simultaneous publications by the Blechert[14] and Hoveyda[15] laboratories. Blechert's name is not commonly included in the eponymous catalyst name. The Hoveyda–Grubbs catalysts, while more expensive and slower to initiate than the Grubbs catalyst from which they are derived, are popular because of their improved stability.[3] Hoveyda–Grubbs catalysts are easily formed from the corresponding Grubbs catalyst by the addition of the chelating ligand and the use of a phosphine scavenger like copper(I) chloride:[15]
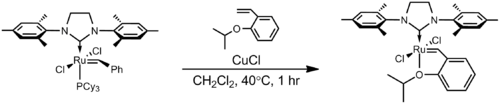
The 2nd generation Hoveyda–Grubbs catalysts can also be prepared from the 1st generation Hoveyda–Grubbs catalyst by the addition of the NHC:[14]

In one study a water soluble Grubbs catalyst is prepared by attaching a polyethylene glycol chain to the imidazolidine group.[16] This catalyst is used in the ring-closing metathesis reaction in water of a diene carrying an ammonium salt group making it water-soluble as well.
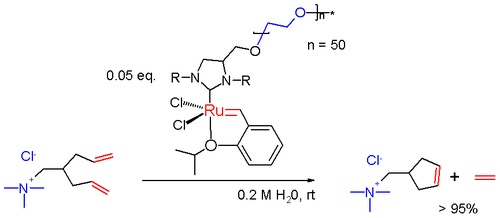
Fast-Initiating Catalysts
The initiation rate of the Grubbs' catalyst can be altered by replacing the phosphine ligand with more labile pyridine ligands. By using 3-bromopyridine the initiation rate is increased more than a million fold:[17]
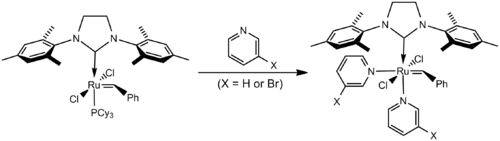
The principle application of the fast-initiating catalysts is as initiators for ring opening metathesis polymerisation (ROMP). Because of their usefulness in ROMP these catalysts are sometimes referred to as the 3rd generation Grubbs' catalysts.[18] The high ratio of the rate of initiation to the rate of propagation makes these catalysts useful in living polymerization, yielding polymers with low polydispersity(see 10.1002/anie.200250632).
Applications
Olefin metathesis is a reaction between two molecules containing double bonds. The groups bonded to the carbon atoms of the double bond are exchanged between molecules, to produce two new molecules containing double bonds with swapped groups. Whether a cis isomer or trans isomer is formed in this type of reaction is determined by the orientation the molecules assume when they coordinate to the catalyst, as well as the sterics of the substituents on the double bond of the newly forming molecule.
On October 5, 2005, Robert H. Grubbs, Richard R. Schrock and Yves Chauvin won the Nobel Prize in Chemistry in recognition of their contributions to the development of this widely used process.
References
- ↑ Grubbs, Robert H. (2003). Handbook of Metathesis (1st ed.). German: Wiley-VCH. ISBN 3-527-30616-1.
- ↑ Grubbs, R.H.; Trnka, T.M.: Ruthenium-Catalyzed Olefin Metathesis in "Ruthenium in Organic Synthesis" (S.-I. Murahashi, Ed.), Wiley-VCH, Germany, 2004.
- ↑ 3.0 3.1 3.2 Vougioukalakis, G. C.; Grubbs, R. H. (2010). "Ruthenium-Based Heterocyclic Carbene-Coordinated Olefin Metathesis Catalysts". Chemical Reviews 110 (3): 1746–1787. doi:10.1021/cr9002424. PMID 20000700.
- ↑ Trnka, T. M.; Grubbs, R. H. (2001). "The Development of L2X2Ru=CHR Olefin Metathesis Catalysts: An Organometallic Success Story". Accounts of Chemical Research 34 (1): 18–29. doi:10.1021/ar000114f. PMID 11170353.
- ↑ Cossy, Janine; Arseniyadis, Stellios; Meyer, Christophe (2010). Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysts (1st ed.). Germany: Wiley-VCH. ISBN 3-527-32440-2.
- ↑ Nguyen, S.T.; Johnson, L.K.; Grubbs, R.H.; Ziller, J.W. (1992). "Ring-opening metathesis polymerization (ROMP) of norbornene by a Group VIII carbene complex in protic media". Journal of the American Chemical Society 114 (10): 3974–3975. doi:10.1021/ja00036a053.
- ↑ Schwab, P.; France, M.B.; Ziller, J.W.; Grubbs, R.H. "A Series of Well-Defined Metathesis Catalysts - Synthesis of [RuCl2(=CHR')(PR3)2] and Its Reactions". Angewandte Chemie-International Edition in English 34 (18): 2039–2041. doi:10.1002/anie.199520391.
- ↑ Schwab, P.; Grubbs, R. H.; Ziller, J. W. (1996). "Synthesis and Applications of RuCl2(=CHR')(PR3)2: The Influence of the Alkylidene Moiety on Metathesis Activity". Journal of the American Chemical Society 118 (1): 100–110. doi:10.1021/ja952676d.
- ↑ Huang, J.-K.; Stevens, E.D.; Nolan, S.P.; Petersen, J.L. (1999). "Olefin Metathesis-Active Ruthenium Complexes Bearing a Nucleophilic Carbene Ligand". Journal of the American Chemical Society 121 (12): 2674–2678. doi:10.1021/ja9831352.
- ↑ Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H. (1999). "Increased Ring Closing Metathesis Activity of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with Imidazolin-2-ylidene Ligands". Tetrahedron Letters 40 (12): 2247–2250. doi:10.1016/S0040-4039(99)00217-8.
- ↑ Ackermann, L.; Fürstner, A.; Weskamp, T.; Kohl, F.J.; Herrmann, W.A. (1999). "Ruthenium Carbene Complexes with Imidazolin-2-ylidene Ligands Allow the Formation of Tetrasubstituted Cycloalkenes by RCM". Tetrahedron Letters 40 (26). doi:10.1016/S0040-4039(99)00919-3.
- ↑ Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. (1999). "Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands". Organic Letters 1 (6): 953–956. doi:10.1021/ol990909q.
- ↑ Kingsbury, Jason S.; Harrity, Joseph P. A.; Bonitatebus, Peter J.; Hoveyda, Amir H. (1999-02-01). "A Recyclable Ru-Based Metathesis Catalyst". Journal of the American Chemical Society 121 (4): 791–799. doi:10.1021/ja983222u.
- ↑ 14.0 14.1 Gessler, S.; Randl, S.; Blechert, S. (2000). "Synthesis and metathesis reactions of phosphine-free dihydroimidazole carbene ruthenium complex". Tetrahedron Letters 41 (51): 9973–9976. doi:10.1016/S0040-4039(00)01808-6.
- ↑ 15.0 15.1 Garber, S.B.; Kingsbury, J.S.; Gray, B.L.; Hoveyda, A.H. (2000). "Efficient and Recyclable Monomeric and Dendritic Ru-Based Metathesis Catalysts". Journal of the American Chemical Society 122 (34): 8168–8179. doi:10.1021/ja001179g.
- ↑ Soon Hyeok Hong and Robert H. Grubbs (2006). "Highly Active Water-Soluble Olefin Metathesis Catalyst". Journal of the American Chemical Society 128 (11): 3508–3509. doi:10.1021/ja058451c. PMID 16536510.
- ↑ Love, J.A.; Morgan, J.P.; Trnka, T.M.; Grubbs, R.H. (2002). "A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile". Angewandte Chemie-International Edition in English 41 (21): 4035–4037. doi:10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I.
- ↑ Leitgeb, Anita; Wappel, Julia; Slugovc, Christian (2010). "The ROMP toolbox upgraded". Polymer 51 (14): 2927–2946. doi:10.1016/j.polymer.2010.05.002.
| ||||||||||||||||||||||||||