Graphite oxide
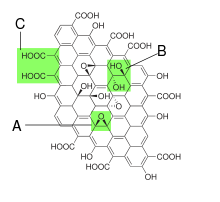
Graphite oxide, formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.[4]
The bulk material disperses in basic solutions to yield monomolecular sheets, known as graphene oxide by analogy to graphene, the single-layer form of graphite.[5] Graphene oxide sheets have been used to prepare a strong paper-like material, and have recently attracted substantial interest as a possible intermediate for the manufacture of graphene. However, this goal remained elusive until 2012 since graphene obtained by this route still has many chemical and structural defects.
History and preparation
Graphite oxide was first prepared by Oxford chemist Benjamin C. Brodie in 1859, by treating graphite with a mixture of potassium chlorate and fuming nitric acid.[6] In 1957 Hummers and Offeman developed a safer, quicker, and more efficient process, using a mixture of sulfuric acid H2SO4, sodium nitrate NaNO3, and potassium permanganate KMnO4, which is still widely used, often with some modifications. [4][7]
It should be noted that graphite oxides demonstrate considerable variations of properties depending on degree of oxidation and synthesis method. For example, temperature point of explosive exfoliation is generally higher for graphite oxide prepared by Brodie method compared to Hummers graphite oxide, the difference is up to 100 degrees with the same heating rates.[8] Hydration and solvation properties of Brodie and Hummers graphite oxides are also remarkably different.[9]
Recently a mixture of H2SO4 and KMnO4 has been used to cut open carbon nanotubes lengthwise, resulting in microscopic flat ribbons of graphene, a few atoms wide, with the edges "capped" by oxygen atoms (=O) or hydroxyl groups (-OH).[10]
Graphite oxide has also been prepared by using a "bottom-up" synthesis method (Tang-Lau method) in which the sole source is glucose, the process is safer, more easy, and more environmentally friendly compared to traditionally “top-down” method, in which strong oxidizers must be involved. Another important advantage of Tang-Lau method is thickness controllable ranging from monolayer to multilayers by simply adjusting growth parameters.[11]
Structure
The structure and properties of graphite oxide depend on particular synthesis method and degree of oxidation. It typically preserves the layer structure of the parent graphite, but the layers are buckled and the interlayer spacing is about two times larger (~0.7 nm) than that of graphite. Strictly speaking "oxide" is an incorrect but historically established name. Besides oxygen epoxide groups (bridging oxygen atoms), other functional groups experimentally found are: carbonyl (=CO), hydroxyl (-OH), phenol and organosulfate groups attached to both sides.[12][13][14] There is evidence of "buckling" (deviation from planarity), folding and cracking [15] of graphene oxide sheets upon deposition of the layers on a choice of substrate. The detailed structure is still not understood due to the strong disorder and irregular packing of the layers.
Graphene oxide layers are about 1.1 ± 0.2 nm thick.[12][13] Scanning tunneling microscopy shows the presence of local regions where oxygen atoms are arranged in a rectangular pattern with lattice constant 0.27 nm × 0.41 nm [13][16] The edges of each layer are terminated with carboxyl and carbonyl groups.[12] X-ray photoelectron spectroscopy shows presence of several C1s peaks, their number and relative intensity depending on particular oxidation method used. Assignment of these peaks to certain carbon functionalization types is somewhat uncertain and still under debates. For example, one of interpretations goes as following: non-oxygenated ring contexts (284.8 eV), C-O (286.2 eV), C=O (287.8 eV) and O-C=O (289.0 eV).[17] Another interpretation using density functional theory calculation goes as following: C=C with defects such as functional groups and pentagons (283.6 eV), C=C (non-oxygenated ring contexts) (284.3 eV), sp3C-H in the basal plane and C=C with functional groups (285.0 eV), C=O and C=C with functional groups, C-O (286.5 eV), and O-C=O (288.3 eV).[18]
Graphite oxide is hydrophilic and easily hydrated exposed to water vapor or immersed in liquid water, resulting in a distinct increase of the inter-planar distance (up to 1.2 nm in saturated state). Additional water is also incorporated into interlayer space due to high pressure induced effects.[19] Maximal hydration state of graphite oxide in liquid water corresponds to insertion of 2-3 water monolayers, cooling the graphite oxide/H2O samples results in "pseudo-negative thermal expansion" and below freezing point of water media results in de-insertion of one water monolayer and lattice contraction. [20] Complete removal of water from the structure seems difficult since heating at 60–80 °C results in partial decomposition and degradation of the material.
Similar to water, graphite oxide also easily incorporates other polar solvents, e.g. alcohols. However, intercalation of polar solvents occurs significantly different in Brodie and Hummers graphite oxides. Brodie graphite oxide is intercalated at ambient conditions with by one monolayer of alcohols and several other solvents (e.g. dimethylformamide and acetone) when liquid solvent is available in excess. Separation of graphite oxide layers is proportional to the size of alcohol molecule.[21] Cooling of Brodie graphite oxide immersed in excess of liquid methanol, ethanol, acetone and dimethylformamide results in step-like insertion of additional solvent monolayer and lattice expansion. The phase transition detected by X-ray diffraction and DSC is reversible; de-insertion of solvent monolayer is observed when sample is heated back from low temperatures. [22] Additional methanol and ethanol monolayer is reversibly inserted into the structure of Brodie graphite oxide also at high pressure conditions.[21]
Hummers graphite oxide is intercalated with two methanol or ethanol monolayers already at ambient temperature. The interlayer distance of Hummers graphite oxide in excess of liquid alcohols increases gradually upon temperature decrease, reaching 19.4 and 20.6 Å at 140 K for methanol and ethanol, respectively. The gradual expansion of the Hummers graphite oxide lattice upon cooling corresponds to insertion of at least two additional solvent monolayers. [23]
Graphite oxide exfoliates and decomposes when rapidly heated at moderately high temperatures (~280–300 °C) with formation of finely dispersed amorphous carbon, somewhat similar to activated carbon.[3]
Applications
Graphene manufacture
Graphite oxide has attracted much interest recently as a possible route for the large-scale production and manipulation of graphene, a material with extraordinary electronic properties. Graphite oxide itself is an insulator,[24] almost a semiconductor, with differential conductivity between 1 and 5×10−3 S/cm at a bias voltage of 10 V.[24] However, being hydrophilic, graphite oxide disperses readily in water, breaking up into macroscopic flakes, mostly one layer thick. Chemical reduction of these flakes would yield a suspension of graphene flakes. It was argued that the first experimental observation of graphene was reported by Hanns-Peter Boehm in 1962.[8] In this early work the existence of monolayer reduced graphene oxide flakes was demonstrated. The contribution of Boehm was recently acknowledged by Andre Geim, the Nobel Prize winner for graphene research.[25]
Partial reduction can be achieved by treating the suspended graphene oxide with hydrazine hydrate at 100 °C for 24 hours,[17] by exposing graphene oxide to hydrogen plasma for a few seconds,[24] or by exposure to a strong pulse of light, such as that of a Xenon flash.[26] Due to the oxidation protocol, manifold defects already present in graphene oxide hamper the effectiveness of the reduction. Thus, the graphene quality obtained after reduction is limited by the precursor quality (graphene oxide) and the efficiency of the reducing agent. [27] However, the conductivity of the graphene obtained by this route is below 10 S/cm,[26] and the charge mobility is between 0.1 and 10 cm2/Vs.[24][28][29] These values are much greater than the oxide's, but still a few orders of magnitude lower than those of pristine graphene.[24] Recently, the synthetic protocol for graphite oxide was optimized and almost intact graphene oxide with a preserved carbon framweork was obtained. Reduction of this almost intact graphene oxide performs much better and the mobility values of charge carriers exceeds 1000 cm2/Vs for the best quality of flakes. [30] Inspection with the atomic force microscope shows that the oxygen bonds distort the carbon layer, creating a pronounced intrinsic roughness in the oxide layers which persists after reduction. These defects also show up in Raman spectra of graphene oxide.[24]
Large amounts of graphene sheets may also be produced through thermal methods. For example, in 2006 a method was discovered that simultaneously exfoliates and reduces graphite oxide by rapid heating (>2000 °C/min) to 1050°C. At this temperature, carbon dioxide is released as the oxygen functionalities are removed and explosively separates the sheets as it comes out.[31]
Additionally, exposing a film of graphite oxide to the laser of a LightScribe DVD has also revealed to produce quality graphene at a low cost. [32]
Water purification
One of suggested applications of graphite oxides is water treatment to remove undesirable pollutants.[33] Brodie graphite oxide is demonstrated to absorb selective methanol from water/methanol mixtures in certain range of methanol concentrations.[34]
Related materials
Dispersed graphene oxide flakes can also be sifted out of the dispersion (as in paper manufacture) and pressed to make an exceedingly strong graphene oxide paper.[11]
Graphene oxide has been used in DNA analysis applications. The large planar surface of graphene oxide allows simultaneous quenching of multiple DNA probes labeled with different dyes, providing the detection of multiple DNA targets in the same solution. Further advances in graphene oxide based DNA sensors could result in very inexpensive rapid DNA analysis. [35]
See also
References
- ↑ He, H.; Klinowski, J.; Forster, M.; Lerf, A. (1998). "A new structural model for graphite oxide". Chemical Physics Letters 287: 53. Bibcode:1998CPL...287...53H. doi:10.1016/S0009-2614(98)00144-4.
- ↑ Log in om een reactie te plaatsen (2011-02-03). "Graphite oxide exfoliation by heating: exlplosion with fire". YouTube. Retrieved 2013-03-18.
- ↑ 3.0 3.1 Talyzin, A. V.; Szabó, T. S.; DéKáNy, I.; Langenhorst, F.; Sokolov, P. S.; Solozhenko, V. L. (2009). "Nanocarbons by High-Temperature Decomposition of Graphite Oxide at Various Pressures". The Journal of Physical Chemistry C 113 (26): 11279. doi:10.1021/jp9016272.
- ↑ 4.0 4.1 Hummers, W. S.; Offeman, R. E. (1958). "Preparation of Graphitic Oxide". Journal of the American Chemical Society 80 (6): 1339. doi:10.1021/ja01539a017.
- ↑ Dreyer, D. R.; Park, S.; Bielawski, C. W.; Ruoff, R. S. (2010). "The chemistry of graphene oxide". Chemical Society Reviews 39 (1): 228–240. doi:10.1039/b917103g. PMID 20023850.
- ↑ Brodie, B. C. (1859). "On the Atomic Weight of Graphite". Philosophical Transactions of the Royal Society of London 149: 249. doi:10.1098/rstl.1859.0013. JSTOR 108699.
- ↑ Marcano, D. C.; Kosynkin, D. V.; Berlin, J. M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L. B.; Lu, W.; Tour, J. M. (2010). "Improved Synthesis of Graphene Oxide". ACS Nano 4 (8): 4806–4814. doi:10.1021/nn1006368. PMID 20731455.
- ↑ 8.0 8.1 Boehm, H. -P.; Scholz, W. (1965). "Der ?Verpuffungspunkt? Des Graphitoxids". Zeitschrift f�r anorganische und allgemeine Chemie 335: 74. doi:10.1002/zaac.19653350107.
- ↑ You, S.; Luzan, S. M.; Szabó, T. S.; Talyzin, A. V. (2013). "Effect of synthesis method on solvation and exfoliation of graphite oxide". Carbon 52: 171. doi:10.1016/j.carbon.2012.09.018.
- ↑ Kosynkin, D. V.; Higginbotham, A. L.; Sinitskii, A.; Lomeda, J. R.; Dimiev, A.; Price, B. K.; Tour, J. M. (2009). "Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons". Nature 458 (7240): 872–876. doi:10.1038/nature07872. PMID 19370030.
- ↑ 11.0 11.1 Tang, L.; Li, X.; Ji, R.; Teng, K. S.; Tai, G.; Ye, J.; Wei, C.; Lau, S. P. (2012). "Bottom-up synthesis of large-scale graphene oxide nanosheets". Journal of Materials Chemistry 22 (12): 5676. doi:10.1039/C2JM15944A.
- ↑ 12.0 12.1 12.2 Schniepp, H. C.; Li, J. L.; McAllister, M. J.; Sai, H.; Herrera-Alonso, M.; Adamson, D. H.; Prud'Homme, R. K.; Car, R.; Saville, D. A.; Aksay, I. A. (2006). "Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide". The Journal of Physical Chemistry B 110 (17): 8535–8539. doi:10.1021/jp060936f. PMID 16640401.
- ↑ 13.0 13.1 13.2 Pandey, D.; Reifenberger, R.; Piner, R. (2008). "Scanning probe microscopy study of exfoliated oxidized graphene sheets". Surface Science 602 (9): 1607. doi:10.1016/j.susc.2008.02.025.
- ↑ Eigler, S.; Dotzer, C.; Hof, F.; Bauer, W.; Hirsch, A. (2013). "Sulfur Species in Graphene Oxide". Chemistry - A European Journal 19 (29): 9490. doi:10.1002/chem.201300387.
- ↑ Pandey, D. K.; Chung, T. F.; Prakash, G.; Piner, R.; Chen, Y. P.; Reifenberger, R. (2011). "Folding and cracking of graphene oxide sheets upon deposition". Surface Science 605 (17–18): 1669. doi:10.1016/j.susc.2011.04.034.
- ↑ Mkhoyan, K. A.; Contryman, A. W.; Silcox, J.; Stewart, D. A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. (2009). "Atomic and Electronic Structure of Graphene-Oxide". Nano Letters 9 (3): 1058–1063. doi:10.1021/nl8034256. PMID 19199476.
- ↑ 17.0 17.1 Stankovich, S.; Piner, R. D.; Chen, X.; Wu, N.; Nguyen, S. T.; Ruoff, R. S. (2006). "Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate)". Journal of Materials Chemistry 16 (2): 155. doi:10.1039/b512799h.
- ↑ Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. (2013). "Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy". Journal of Materials Science 48 (23): 8171. doi:10.1007/s10853-013-7630-0.
- ↑ Talyzin, A. V.; Solozhenko, V. L.; Kurakevych, O. O.; Szabó, T. S.; Dékány, I.; Kurnosov, A.; Dmitriev, V. (2008). "Colossal Pressure-Induced Lattice Expansion of Graphite Oxide in the Presence of Water". Angewandte Chemie International Edition 47 (43): 8268. doi:10.1002/anie.200802860.
- ↑ You, S.; Luzan, S. M.; Szabó, T. S.; Talyzin, A. V. (2013). "Effect of synthesis method on solvation and exfoliation of graphite oxide". Carbon 52: 171. doi:10.1016/j.carbon.2012.09.018.
- ↑ 21.0 21.1 Talyzin, A. V.; Sundqvist, B.; Szabó, T. S.; DéKáNy, I.; Dmitriev, V. (2009). "Pressure-Induced Insertion of Liquid Alcohols into Graphite Oxide Structure". Journal of the American Chemical Society 131 (51): 18445–18449. doi:10.1021/ja907492s. PMID 19947629.
- ↑ You, S.; Luzan, S.; Yu, J.; Sundqvist, B.; Talyzin, A. V. (2012). "Phase Transitions in Graphite Oxide Solvates at Temperatures Near Ambient". The Journal of Physical Chemistry Letters 3 (7): 812. doi:10.1021/jz300162u.
- ↑ You, S.; Sundqvist, B.; Talyzin, A. V. (2013). "Enormous Lattice Expansion of Hummers Graphite Oxide in Alcohols at Low Temperatures". ACS Nano 7 (2): 1395–1399. doi:10.1021/nn3051105. PMID 23297717.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 Gómez-Navarro, C.; Weitz, R. T.; Bittner, A. M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. (2007). "Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets". Nano Letters 7 (11): 3499–3503. doi:10.1021/nl072090c. PMID 17944526.
- ↑ "Letters to the Editor". APS News (American Physical Society) 19 (1). January 2010.
- ↑ 26.0 26.1 Cote, L. J.; Cruz-Silva, R.; Huang, J. (2009). "Flash Reduction and Patterning of Graphite Oxide and Its Polymer Composite". Journal of the American Chemical Society 131 (31): 11027–11032. doi:10.1021/ja902348k. PMID 19601624.
- ↑ Eigler, S.; Grimm, S.; Enzelberger-Heim, M.; Müller, P.; Hirsch, A. (2013). "Graphene oxide: Efficiency of reducing agents". Chemical Communications 49 (67): 7391–7393. doi:10.1039/C3CC43612H. PMID 23860424.
- ↑ GóMez-Navarro, C.; Weitz, R. T.; Bittner, A. M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. (2009). "Electronic Transport Properties of Individual Chemically Reduced Graphene Oxide Sheets". Nano Letters 9 (5): 2206. doi:10.1021/nl901209z.
- ↑ Eda, G.; Ball, J.; Mattevi, C.; Acik, M.; Artiglia, L.; Granozzi, G.; Chabal, Y.; Anthopoulos, T. D.; Chhowalla, M. (2011). "Partially oxidized graphene as a precursor to graphene". Journal of Materials Chemistry 21 (30): 11217. doi:10.1039/C1JM11266J.
- ↑ Eigler, S.; Enzelberger-Heim, M.; Grimm, S.; Hofmann, P.; Kroener, W.; Geworski, A.; Dotzer, C.; Röckert, M.; Xiao, J.; Papp, C.; Lytken, O.; Steinrück, H. P.; Müller, P.; Hirsch, A. (2013). "Wet Chemical Synthesis of Graphene". Advanced Materials 25 (26): 3583–3587. doi:10.1002/adma.201300155. PMID 23703794.
- ↑ Schniepp, H. C., Li, J.-L., McAllister, M. J., Sai, H., Herrera-Alonso, M., Adamson, D. H., … Aksay, I. a. (2006). Functionalized single graphene sheets derived from splitting graphite oxide. The journal of physical chemistry. B, 110(17), 8535–9. doi:10.1021/jp060936f
- ↑ El-Kady, M. F.; Strong, V.; Dubin, S.; Kaner, R. B. (2012). "Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors". Science 335 (6074): 1326–1330. doi:10.1126/science.1216744. PMID 22422977.
- ↑ Gao, W.; Majumder, M.; Alemany, L. B.; Narayanan, T. N.; Ibarra, M. A.; Pradhan, B. K.; Ajayan, P. M. (2011). "Engineered Graphite Oxide Materials for Application in Water Purification". ACS Applied Materials & Interfaces 3 (6): 1821. doi:10.1021/am200300u.
- ↑ You, S.; Yu, J.; Sundqvist, B.; Belyaeva, L. A.; Avramenko, N. V.; Korobov, M. V.; Talyzin, A. V. (2013). "Selective Intercalation of Graphite Oxide by Methanol in Water/Methanol Mixtures". The Journal of Physical Chemistry C 117 (4): 1963. doi:10.1021/jp312756w.
- ↑ He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. (2010). "A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis". Advanced Functional Materials 20 (3): 453. doi:10.1002/adfm.200901639.
| ||||||||||||||
