Gramine
| Gramine | |
|---|---|
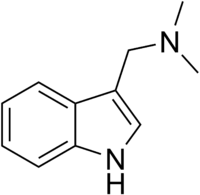 | |
| IUPAC name 1-(1H-indol-3-yl)-N,N-dimethylmethanamine | |
| Identifiers | |
| CAS number | 87-52-5 |
| ChemSpider | 6625 |
| KEGG | C08304 |
| ChEMBL | CHEMBL254348 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C11H14N2 |
| Molar mass | 174.24 g/mol |
| Melting point | 138-139 °C |
| Hazards | |
| NFPA 704 |
 1
2
0
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Gramine (also called donaxine) is a naturally occurring indole alkaloid present in several plant species. Gramine may play a defensive role in these plants, since it is toxic to many organisms.[1]
Occurrence
Gramine has been found in the giant reed, Arundo donax,[2][3]Acer saccharinum (Silver Maple),[4] Hordeum,[1][3] and Phalaris[3] plant species.
Synthesis
Despite being widely available in several plant species, gramine is far easier to synthesize directly from indole via a Mannich reaction with dimethylamine and formaldehyde.
Uses
Gramine is used mostly in synthetic organic chemistry as a starting material for tryptophan syntheses.
All reactions of gramine follow the same general reaction scheme. Gramine is reacted with a strong electrophile, such as methyl iodide, to form the quaternary ammonium salt 2. The ammonium salt will undergo a Hofmann elimination or Michael addition to give the very active intermediate 3, which can accept a wide range of nucleophiles to give the desired product 4.
Toxicity
The LD50 of gramine is 44.6 mg/ kg iv in mice and 62.9 mg/ kg iv in rats.[5] Numerous studies have been done on the toxicity in insects harmful to crops for use as a possible insecticide.[6]
Gramine is a norepinephrine reuptake inhibitor in synaptic vesicles.[citation needed] Resulting effects of extra norepinephrine raises blood pressure and heart rate.
References
- ↑ 1.0 1.1 Corcuera, L. J. (1993). "Biochemical Basis of the Resistance of the Barley to Aphids". Phytochemistry 33 (4): 741–747. doi:10.1016/0031-9422(93)85267-U.
- ↑ Orechoff, A.; Norkina, S. (1935). "Über die Alkaloide von Arundo Donax L.". Berichte der Deutschen Chemischen Gesellschaft 68 (3): 436–437. doi:10.1002/cber.19350680312.
- ↑ 3.0 3.1 3.2 Cheeke, P. R. (1989). Toxicants of Plant Origin, Alkaloids. CRC Press. p. 172. ISBN 0-8493-6990-8.
- ↑ Pachter, I. J.; Zacharias, D. E.; Ribeiro, O. (1959). "Indole Alkaloids of Acer saccharinum (the Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis". Journal of Organic Chemistry 24 (9): 1285–1287. doi:10.1021/jo01091a032.
- ↑ Erspamer, V. (1954). "Pharmacology of Indolealkylamines". Pharmacological Reviews 6 (4): 425–487. PMID 13236482.
- ↑ Corcuera, L. J. (1984). "Effects of Indole Alkaloids from Gramineae on Aphids". Phytochemistry 23 (3): 539–541. doi:10.1016/S0031-9422(00)80376-3.

