Gold(III) fluoride
From Wikipedia, the free encyclopedia
| Gold(III) fluoride[1] | |
|---|---|
 | |
| IUPAC name Gold(III) fluoride | |
| Other names Gold trifluoride | |
| Identifiers | |
| CAS number | 14720-21-9 |
| PubChem | 5460532 |
| ChemSpider | 10790539 |
| ChEBI | CHEBI:30077 |
| Jmol-3D images | {{#if:[Au+3].[F-].[F-].[F-]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | AuF3 |
| Molar mass | 253.962 g/mol |
| Appearance | orange-yellow hexagonal crystals |
| Density | 6.75 g/cm3 |
| Melting point | sublimes above 300°C |
| Structure | |
| Crystal structure | Hexagonal, hP24 |
| Space group | P6122, No. 178 |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
-363.3 kJ/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Gold(III) fluoride, AuF3, is an orange solid that sublimes at 300 °C.[2] It is a powerful fluorinating agent.
Preparation
AuF3 can be prepared by reacting AuCl3 with F2 or BrF3.
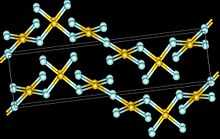
Structure
The crystal structure of AuF3 consists of spirals of square-planar AuF4 units.[3]
 |  |  |  |  |
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–59. ISBN 0-8493-0594-2.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419., p. 1184.
- ↑ F. W. B. Einstein, P. R. Rao, James Trotter and Neil Bartlett (1967). "The crystal structure of gold trifluoride". Journal of the Chemical Society A: Inorganic, Physical, Theoretical 4: 478–482. doi:10.1039/J19670000478.
| ||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.