Gmelanone
From Wikipedia, the free encyclopedia
| Gmelanone | ||
|---|---|---|
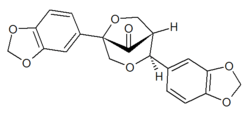 | ||
| IUPAC name (1R,2S,5S)-2,5-Bis(1,3-benzodioxol-5-yl)-3,6-dioxabicyclo[3.2.1]octan-8-one | ||
| Identifiers | ||
| ChemSpider | 10308036 | |
| Jmol-3D images | {{#if:O=C4[C@@H]1CO[C@]4(CO[C@@H]1c2ccc3OCOc3c2)c5ccc6OCOc6c5|Image 1 | |
| ||
| Properties | ||
| Molecular formula | C20H16O7 | |
| Molar mass | 368.33 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Gmelanone is a lignan found in the heartwood of Gmelina arborea.[1]
Arboreol can be transformed by acid catalysis into gmelanone.[2]
References
- ↑ Novel hydroxy lignans from the heartwood of gmelina arborea. A.S.R. Anjaneyulu, A.Madhusudhana rao, V.Kameswara Rao and L.Ramachandra Row, Tetrahedron, 1977, Volume 33, Issue 1, Pages 133–143, doi:10.1016/0040-4020(77)80444-4
- ↑ Acid catalysed rearrangements of arboreol: A biomimetic synthesis of gmelanone. L. Ramachandra Row and Reveru Ventkateswarlu, Tetrahedron Letters, 1980, Volume 21, Issue 30, Pages 2919–2922, doi:10.1016/S0040-4039(00)78645-X
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.