Glyceollin I
From Wikipedia, the free encyclopedia
| Glyceollin I | ||
|---|---|---|
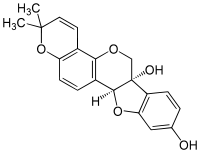 | ||
| IUPAC name (6aS,11aS)-2,2-dimethyl-2H,6H-[1]benzofuro[3,2‑c]pyrano[2,3‑h] | ||
| Other names (−)-Glyceollin I | ||
| Identifiers | ||
| CAS number | 57103-57-8 | |
| PubChem | 162807 | |
| Properties | ||
| Molecular formula | C20H18O5 | |
| Molar mass | 338 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.[1]
Glyceollin synthase is an enzyme responsible for the production of glyceollin.[2] The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, NADPH, H+, and O2, whereas its three products are glyceollin, NADP+, and H2O.
In in vitro studies, this molecule has been shown to exhibit antiestrogenic properties.[3]
References
- ↑ Zimmermann, M. C.; Tilghman, S. L.; Boué, S. M.; Salvo, V. A.; Elliott, S.; Williams, K. Y.; Skripnikova, E. V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; Fonseca, J. P.; Corbitt, C.; Collins-Burow, B. M.; Howell, M. H.; Lacey, M.; Shih, B. Y.; Carter-Wientjes, C.; Cleveland, T. E.; McLachlan, J. A.; Wiese, T. E.; Beckman, B. S.; Burow, M. E. (2009). "Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy". Journal of Pharmacology and Experimental Therapeutics 332 (1): 35–45. doi:10.1124/jpet.109.160382. PMC 2802480. PMID 19797619.
- ↑ Welle, R.; Grisebach, H. (1988). "Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers". Archives of Biochemistry and Biophysics 263 (1): 191–198. doi:10.1016/0003-9861(88)90627-3. PMID 3369863.
- ↑ Payton-Stewart, F.; Khupse, R. S.; Boué, S. M.; Elliott, S.; Zimmermann, M. C.; Skripnikova, E. V.; Ashe, H.; Tilghman, S. L.; Beckman, B. S.; Cleveland, T. E.; McLachlan, J. A.; Bhatnagar, D.; Wiese, T. E.; Erhardt, P.; Burow, M. E. (2010). "Glyceollin I enantiomers distinctly regulate ER-mediated gene expression". Steroids 75 (12): 870–878. doi:10.1016/j.steroids.2010.05.007. PMID 20493896.
| |||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.