Glipizide
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
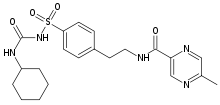
| N-(4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenethyl)-5-methylpyrazine-2-carboxamide | |
| Clinical data | |
| Trade names | Glucotrol |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a684060 |
| Pregnancy cat. | C (Au, U.S.) |
| Legal status | POM (UK), ℞-only (U.S.) |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 100% (regular formulation) 90% (extended release) |
| Protein binding | 98 to 99% |
| Metabolism | Hepatic hydroxylation |
| Half-life | 2 to 5 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| CAS number | 29094-61-9 |
| ATC code | A10BB07 |
| PubChem | CID 3478 |
| DrugBank | DB01067 |
| ChemSpider | 3359 |
| UNII | X7WDT95N5C |
| KEGG | D00335 |
| ChEMBL | CHEMBL1073 |
| Chemical data | |
| Formula | C21H27N5O4S |
| Mol. mass | 445.536 g/mol |
| SMILES
| |
| |
| | |
Glipizide is an oral rapid- and short-acting anti-diabetic drug from the sulfonylurea class. It is classified as a second generation sulfonylurea, which means that it undergoes enterohepatic circulation. Second-generation sulfonylureas are both more potent and have shorter half-lives than the first-generation sulfonylureas.
Mechanism of action is produced by blocking potassium channels in the beta cells of the islets of Langerhans. By partially blocking the potassium channels, the cell remains depolarized, increasing the time the cell spends in the calcium release stage, which results in signaling leading to calcium influx. The increase in calcium will initiate more insulin release from each beta cell. Sulfonylureas may also cause the decrease of serum glucagon and potentiate the action of insulin at the extrapancreatic tissues.
Originally available in 1984, it is marketed by Pfizer under the brand name Glucotrol in the USA, where Pfizer sells Glucotrol in doses of 5 and 10 milligrams and Glucotrol XL (an extended release form of glipizide) in doses of 2.5, 5, and 10 milligrams. Other companies also market glipizide, most commonly extended release tablets of 5 and 10 milligrams.
External links
- Glucotrol XL Full U.S. Prescribing Information. Accessed on July 26, 2005.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||