Glioma
| Glioma | |
|---|---|
| Classification and external resources | |
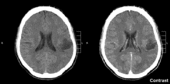 Glioma in the left parietal lobe (brain CT scan), WHO grade 2. | |
| ICD-10 | C71 |
| ICD-9 | 191 |
| ICD-O: | M9380/3-9460/3 |
| DiseasesDB | 31468 |
| MeSH | D005910 |
A glioma is a type of tumor that starts in the brain or spine. It is called a glioma because it arises from glial cells. The most common site of gliomas is the brain.[1] Gliomas make up ~30% of all brain and central nervous system tumors and 80% of all malignant brain tumors.[2]
Classification
Gliomas are classified by cell type, by grade, and by location.
By type of cell
Gliomas are named according to the specific type of cell they share histological features with, but not necessarily originate from. The main types of gliomas are:
- Ependymomas—ependymal cells.
- Astrocytomas—astrocytes (glioblastoma multiforme is the most common astrocytoma).
- Oligodendrogliomas—oligodendrocytes.
- Brainstem glioma — develop in the brain stem
- Optic nerve glioma — develop in or around the optic nerve
- Mixed gliomas, such as oligoastrocytomas, contain cells from different types of glia.
By grade
Gliomas are further categorized according to their grade, which is determined by pathologic evaluation of the tumor.

- Low-grade gliomas [WHO grade II] are well-differentiated (not anaplastic); these tend to exhibit benign tendencies and portend a better prognosis for the patient. However, they have a uniform rate of recurrence and increase in grade over time so should be classified as malignant.
- High-grade [WHO grade III–IV] gliomas are undifferentiated or anaplastic; these are malignant and carry a worse prognosis.
Of numerous grading systems in use, the most common is the World Health Organization (WHO) grading system for astrocytoma, under which tumors are graded from I (least advanced disease—best prognosis) to IV (most advanced disease—worst prognosis).
By location
Gliomas can be classified according to whether they are above or below a membrane in the brain called the tentorium. The tentorium separates the cerebrum (above) from the cerebellum (below).
- supratentorial: above the tentorium, in the cerebrum, mostly found in adults (70%).
- infratentorial: below the tentorium, in the cerebellum, mostly found in children (70%).
- pontine: located in the pons of the brainstem. The brainstem has three parts (pons, midbrain and medulla); the pons controls critical functions such as breathing, making surgery on these extremely dangerous.
Signs and symptoms
Symptoms of gliomas depend on which part of the central nervous system is affected. A brain glioma can cause headaches, nausea and vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure. A glioma of the optic nerve can cause visual loss. Spinal cord gliomas can cause pain, weakness, or numbness in the extremities. Gliomas do not metastasize by the bloodstream, but they can spread via the cerebrospinal fluid and cause "drop metastases" to the spinal cord.
A child who has a subacute disorder of the central nervous system that produces cranial nerve abnormalities (especially of cranial nerve VII and the lower bulbar nerves), long-tract signs, unsteady gait secondary to spasticity, and some behavioral changes is most likely to have a pontine glioma.[3]
Causes
The exact causes of gliomas are not known. Hereditary genetic disorders such as neurofibromatoses (type 1 and type 2) and tuberous sclerosis complex are known to predispose to their development.[4]
Gliomas have been correlated to the electromagnetic radiation from cell phones, and a link between the cancer and cell phone usage is considered plausible, though there is no conclusive evidence.[5] Experiments designed to test such a link gave negative results.[6] Most glioblastomas are infected with cytomegalovirus, however the significance of this is not known.[7][8]
Pathophysiology
High-grade gliomas are highly-vascular tumors and have a tendency to infiltrate. They have extensive areas of necrosis and hypoxia. Often tumor growth causes a breakdown of the blood–brain barrier in the vicinity of the tumor. As a rule, high-grade gliomas almost always grow back even after complete surgical excision, and so are commonly called recurrent cancer of the brain.
On the other hand, low-grade gliomas grow slowly, often over many years, and can be followed without treatment unless they grow and cause symptoms.
Several acquired (not inherited) genetic mutations have been found in gliomas. Tumor suppressor protein 53 (p53) is an early mutation. p53 is the "guardian of the genome," which, during DNA and cell duplication, makes sure that the DNA is copied correctly and destroys the cell (apoptosis) if the DNA is mutated and can't be fixed. When p53 itself is mutated, other mutations can survive. Phosphatase and tensin homolog (PTEN), another protein that also helps destroy cells with dangerous mutations, is itself lost or mutated. Epidermal growth factor receptor (EGFR), a growth factor that normally stimulates cells to divide, is amplified and stimulates cells to divide too much. Together, these mutations lead to cells dividing uncontrollably, a hallmark of cancer. Recently, mutations in IDH1 and IDH2 were found to be part of the mechanism and associated with a more favorable prognosis.[9] The IDH1 and IDH2 genes are significant because they are involved in the citric acid cycle in mitochondria. Mitochondria are involved in apoptosis. Furthermore, the altered glycolysis metabolism in some cancer cells leads to low oxygen (hypoxia). The normal response to hypoxia is to stimulate the growth of new blood vessels (angiogenesis). So these two genes may contribute to both the lack of apoptosis and vascularization of gliomas.
Treatment
Treatment for brain gliomas depends on the location, the cell type and the grade of malignancy. Often, treatment is a combined approach, using surgery, radiation therapy, and chemotherapy. The radiation therapy is in the form of external beam radiation or the stereotactic approach using radiosurgery. Spinal cord tumors can be treated by surgery and radiation. Temozolomide is a chemotherapeutic drug that is able to cross the blood–brain barrier effectively and is currently being used in therapy for high-grade tumors.
Refractory disease
For recurrent high-grade glioblastoma, recent studies have taken advantage of angiogenic blockers such as bevacizumab in combination with conventional chemotherapy, with encouraging results.[10]
Relative effectiveness
A 2007 meta-analysis compared surgical resection and biopsy as the initial surgical management option. Results show that there is insufficient evidence to make a reliable decision.[11] For high-grade gliomas, a 2003 meta-analysis compared radiotherapy with radiotherapy and chemotherapy. It showed a small but clear improvement from using chemotherapy with radiotherapy.[12] In low grade gliomas a comparative study found that treatment a center that favored early surgical resection was associated with better overall survival than treatment at a center that favored biopsy and watchful waiting.[13] For Glioblastoma Multiforme, a 2008 meta-analysis showed that Temozolomide is an effective treatment for "prolonging survival and delaying progression as part of primary therapy without impacting on QoL and with a low incidence of early adverse events."[14]
Prognosis
Gliomas are rarely curable. The prognosis for patients with high-grade gliomas is generally poor, and is especially so for older patients. Of 10,000 Americans diagnosed each year with malignant gliomas, about half are alive one year after diagnosis, and 25% after two years. Those with anaplastic astrocytoma survive about three years. Glioblastoma multiforme has a worse prognosis with less than a 12-month average survival after diagnosis, though this has extended to 14 months with more recent treatments .[15]
Low grade
For low-grade tumors, the prognosis is somewhat more optimistic. Patients diagnosed with a low-grade glioma are 17 times as likely to die as matched patients in the general population.[16] The age-standardized 10-year relative survival rate was 47%.[16] One study reported that low-grade oligodendroglioma patients have a median survival of 11.6 years;[17] another reported a median survival of 16.7 years.[18]
High grade
This group comprises anaplastic astrocytomas and glioblastoma multiforme.
Diffuse intrinsic pontine glioma
Prognosis of this tumour has a very low (about 10-15%) survival rate. Surgery to attempt tumour removal is usually not possible or advisable for DIPG. By their very nature, these tumours invade diffusely throughout the brain stem, growing between normal nerve cells. Aggressive surgery would cause severe damage to neural structures vital for arm and leg movement, eye movement, swallowing, breathing, and even consciousness.[19][20]
References
- ↑ Mamelak AN, Jacoby DB (March 2007). "Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601)". Expert Opin Drug Deliv 4 (2): 175–86. doi:10.1517/17425247.4.2.175. PMID 17335414.
- ↑ Goodenberger ML, Jenkins RB (2012). "Genetics of adult glioma". Cancer Genet. doi:10.1016/j.cancergen.2012.10.009.
- ↑ PRETEST pediatrics pg 224
- ↑ Reuss, D; von Deimling, A (2009). "Hereditary tumor syndromes and gliomas.". Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 171: 83–102. doi:10.1007/978-3-540-31206-2_5. PMID 19322539.
- ↑ http://www.iarc.fr/en/media-centre/pr/2011/pdfs/pr208_E.pdf
- ↑ Benson, Victoria; Kristin Pirie, Joachim Schüz, Gillian K Reeves, Valerie Beral, Jane Green (23 March 2013). "Mobile phone use and risk of brain neoplasms and other cancers: prospective study". International Journal of Epidemiology 42 (3): 792–802. doi:10.1093/ije/dyt072. Retrieved 8 May 2013.
- ↑ Michaelis, M; Baumgarten, P, Mittelbronn, M, Driever, PH, Doerr, HW, Cinatl J, Jr (February 2011). "Oncomodulation by human cytomegalovirus: novel clinical findings open new roads.". Medical microbiology and immunology 200 (1): 1–5. doi:10.1007/s00430-010-0177-7. PMID 20967552.
- ↑ Barami, K (July 2010). "Oncomodulatory mechanisms of human cytomegalovirus in gliomas.". Journal of Clinical Neuroscience 17 (7): 819–23. doi:10.1016/j.jocn.2009.10.040. PMID 20427188.
- ↑ Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (19 Feb 2009). "IDH1 and IDH2 mutations in gliomas". N Engl J Med 360 (8): 765–73. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619.
- ↑ Wong ET, Brem S (2007). "Taming glioblastoma: targeting angiogenesis". J. Clin. Oncol. 25 (30): 4705–6. doi:10.1200/JCO.2007.13.1037. PMID 17947716.
- ↑ Hart MG, Grant R, Metcalfe SE (2000). "Biopsy versus resection for high grade glioma". In Hart, Michael G. Cochrane Database Syst Rev (2): CD002034. doi:10.1002/14651858.CD002034. PMID 10796847.
- ↑ Stewart L, Burdett S; Glioma Meta-analysis Trialists Group (GMT) (2002). "Chemotherapy for high-grade glioma". In Stewart, Lesley. Cochrane Database Syst Rev (3): CD003913. doi:10.1002/14651858.CD003913. PMID 12519620.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/23099483
- ↑ Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K (2008-10-08). "Temozolomide for high grade glioma". In Hart, Michael G. Cochrane Database Syst Rev (4): CD007415. doi:10.1002/14651858.CD007415. PMID 18843749.
- ↑ Rob Stein (May 20, 2008). "Malignant Gliomas Affect About 10,000 Americans Annually". Washington Post.
- ↑ 16.0 16.1 Smoll N, Gautschi OP, Schatlo B, Schaller K, Weber DC (July 6, 2012). "Relative Survival of Patients with Supratentorial Low Grade Gliomas". Neuro-Oncology.
- ↑ Ohgaki H, Kleihues P (June 2005). "Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas". J Neuropathol Exp Neurol. 64 (6): 479–89. PMID 15977639.
- ↑ http://www.neurology.org/cgi/content/abstract/54/7/1442
- ↑ Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, Foer DR, Goldthwaite PT, Burger PC. A clinicopathologic reappraisal of brainstem tumor classification: identification of pilocytic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer 89:1569-1576, 2000.
- ↑ Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brain stem gliomas. J Clin Oncol 24:1266-1272, 2006.
External links
- American Brain Tumor Association: Malignant Gliomas
- Brain and Spinal Tumors: Hope Through Research (National Institute of Neurological Disorders and Stroke)
- -2147090429 at GPnotebook
- German Brain Tumor Association
- WHO Classification of Glioma
- Glioma Images MedPix Database
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||