Gephyrotoxin
| Gephyrotoxin | |
|---|---|
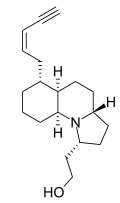 | |
| IUPAC name 2-{(1R,3aR,5aR,6R,9aS)-6-[(2Z)-pent-2-en-4-yn-1-yl]dodecahydropyrrolo[1,2-a]quinolin-1-yl}ethanol | |
| Other names Histrionicotoxin D; HTX D | |
| Identifiers | |
| CAS number | 55893-12-4 |
| PubChem | 6437870 |
| ChemSpider | 4942391 |
| Jmol-3D images | {{#if:C#C\C=C/C[C@@H]3[C@H]2CC[C@H]1N([C@H](CC1)CCO)[C@H]2CCC3|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C19H29NO |
| Molar mass | 287.44 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Gephyrotoxin is a naturally occurring product that stems from the Colombian tropical frog Dendrobates histrionicus. It is a member of the class of compounds known as histrionicotoxins. This alkaloid skin secretion was first isolated from the tropical frog in 1977 by Daly and his fellow workers.[1]
Biological uses
This compound is a relatively non-toxic chemical. At first it showed activity as a slight muscarinic antagonist, but with recent studies it has showed other interesting neurological activities. Due to these new activities, many laboratories want to conduct future research on it. Due to this demand and the scaracity and unabundance of the tree frog the synthesis of this product is of much interest.[1]
Synthesis
The first total synthesis of gephyrotoxin was performed by Kishi and his co-workers, here they prepped an intermediate from L-pyroglutamic acid in 18 steps. Others have made it to the same intermediate in fewer steps but have included poorly diastereoselective steps. In 2008 Santarem and colleges reported total synthesis of Gephyrotoxin by the obtention of an enantionpure cis-2,5-disubstituted pyrrolidine. Unlike others that included a poorly diastereoselective step, this process allowed for the development of two stereogenic centers at the same time.[1][2]
References
- ↑ 1.0 1.1 1.2 Santarem, Marco; Vanucci-Bacqué, Corinne; Lhommet, GéRard (2008). "Formal Total Synthesis of (+)-Gephyrotoxin". The Journal of Organic Chemistry 73 (16): 6466–6469. doi:10.1021/jo801150e. PMID 18637692.
- ↑ Daly, J. W.; Witkop, B.; Tokuyama, T.; Nishikawa, T.; Karle, I. L. (1977). "Gephyrotoxins, Histrionicotoxins and Pumiliotoxins from the Neotropical FrogDendrobates histrionicus". Helvetica Chimica Acta 60 (3): 1128–1140. doi:10.1002/hlca.19770600336. PMID 863724.