Gemifloxacin
 | |
|---|---|
| Systematic (IUPAC) name | |
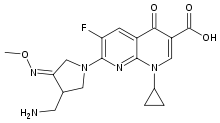
| 7-[(4Z)-3-(Aminomethyl)-4-methoxyimino-pyrrolidin-1-yl]-1-cyclopropyl-6-fluoro-4-oxo- 1,8-naphthyridine-3-carboxylic acid | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a604014 |
| Pregnancy cat. | C |
| Legal status | ℞ Prescription only |
| Routes | Oral/IV under development |
| Pharmacokinetic data | |
| Bioavailability | 71% |
| Protein binding | 60-70% |
| Metabolism | Limited metabolism by the liver to minor metabolites |
| Excretion | Feces (61%); urine (36%) |
| Identifiers | |
| CAS number | 175463-14-6 |
| ATC code | J01MA15 |
| PubChem | CID 9571107 |
| DrugBank | DB01155 |
| ChemSpider | 7845573 |
| UNII | OKR68Y0E4T |
| KEGG | D08012 |
| ChEBI | CHEBI:101853 |
| ChEMBL | CHEMBL430 |
| Chemical data | |
| Formula | C18H20FN5O4 |
| Mol. mass | 389.381 g/mol |
| SMILES
| |
| |
| | |
Gemifloxacin mesylate (trade name Factive, Oscient Pharmaceuticals) is an oral broad-spectrum quinolone antibacterial agent used in the treatment of acute bacterial exacerbation of chronic bronchitis and mild-to-moderate pneumonia. Oscient Pharmaceuticals has licensed the active ingredient from LG Life Sciences of Korea.
Indications
Gemifloxacin is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
- Acute bacterial exacerbation of chronic bronchitis caused by S. pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis
- Community-acquired pneumonia (of mild to moderate severity) caused by S. pneumoniae (including multi-drug resistant strains, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae, or Klebsiella pneumoniae
Microbiology
Gemifloxacin has been shown to be active against most strains of the following microorganisms:
- Anaerobic gram-positive microorganisms - Streptococcus pneumoniae[1]
including multi-drug resistant Streptococcus pneumoniae (MDRSP). MDRSP includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin, 2nd generation cephalosporins, e.g., cefuroxime, macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
- Aerobic gram-negative microorganisms - Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae (many strains are moderately susceptible), Moraxella catarrhalis, Acinetobacter lwoffii, Klebsiella oxytoca, Legionella pneumophila, Proteus vulgaris.
- Other microorganisms - Chlamydia pneumoniae, Mycoplasma pneumoniae
Adverse effects
Fluoroquinolones are generally well tolerated with most side effects being mild and serious adverse effects being rarely.[2][3] Some of the serious adverse effects which occur more commonly with fluoroquinolones than with other antibiotic drug classes include CNS and tendon toxicity.[4][5] The currently marketed quinolones have safety profiles similar to that of other antimicrobial classes.[4]
The serious events may occur with therapeutic or with acute overdose. At therapeutic doses they include: central nervous system toxicity, cardiovascular toxicity, tendon / articular toxicity, and rarely hepatic toxicity.[6] Events that may occur in acute overdose are rare and include: renal failure and seizure.[6] Children and the elderly are at greater risk.[2][5] Tendon damage may manifest during, as well as up to a year after fluoroquinolone therapy.[7]
Some groups refer to these adverse events as "fluoroquinolone toxicity". These groups of people claim to have suffered serious long term harm to their health from using fluoroquinolones. This has led to a class action lawsuit by people harmed by the use of fluoroquinolones as well as action by the consumer advocate group Public Citizen.[8][9] Partly as a result of the efforts of Public Citizen the FDA ordered a black box warnings on all fluoroquinolones advising consumers of the possible toxic effects of fluoroquinolones on tendons.[10]
On August 15th, 2013 the FDA issued a Safety Announcement where they described that they are requiring the medication guides and drug labels for all fluoroquinolones to be updated and better describe the risk for peripheral neuropathy.[11] The peripheral neuropathy may occur very quickly, and may be irreversible. This warning applies to fluoroquinolones taken by mouth and injection, but does not apply to fluoroquinolones taken topically.
See also
- Fluoroquinolone
References
- ↑ Calvo A, Gimenez MJ (2002). "Ex Vivo Serum Activity (Killing Rates) After Gemifloxacin 320 mg Versus Trovafloxacin 200 mg Single Doses Against Ciprofloxacin-Susceptible and -Resistant Streptococcus pneumoniae". Int. J. Antimicrob. Agents 20 (2): 144–6. doi:10.1016/S0924-8579(02)00119-X. PMID 12297365.
- ↑ 2.0 2.1 Owens RC, Ambrose PG (July 2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881.
- ↑ Ball P, Mandell L, Niki Y, Tillotson G (November 1999). "Comparative tolerability of the newer fluoroquinolone antibacterials". Drug Saf 21 (5): 407–21. doi:10.2165/00002018-199921050-00005. PMID 10554054.
- ↑ 4.0 4.1 Owens RC, Ambrose PG (July 2005). "Antimicrobial safety: focus on fluoroquinolones". Clin. Infect. Dis. 41 Suppl 2: S144–57. doi:10.1086/428055. PMID 15942881.
- ↑ 5.0 5.1 Iannini PB (June 2007). "The safety profile of moxifloxacin and other fluoroquinolones in special patient populations". Curr Med Res Opin 23 (6): 1403–13. doi:10.1185/030079907X188099. PMID 17559736.
- ↑ 6.0 6.1 Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R.; Hoffman, Robert Louis; Howland, Mary Deems; Neal A. Lewin (2006). Goldfrank's toxicologic emergencies. New York: McGraw-Hill, Medical Pub. Division. ISBN 0-07-143763-0. OCLC url=http://books.google.com/books?id=cvJuLqBxGUcC&pg=PA849&dq=goldfranks+Fluoroquinolone+toxicity.
- ↑ Saint F, Gueguen G, Biserte J, Fontaine C, Mazeman E (September 2000). "[Rupture of the patellar ligament one month after treatment with fluoroquinolone]". Rev Chir Orthop Reparatrice Appar Mot (in French) 86 (5): 495–7. PMID 10970974.
- ↑ http://www.consumeraffairs.com/news04/2006/08/pubcit_cipro.html
- ↑ http://www.mnd.uscourts.gov/MDL-Levaquin/index.shtml
- ↑ "FDA orders 'black box' label on some antibiotics". CNN. 2008-07-08. Retrieved 2008-07-08.
- ↑ http://www.fda.gov/downloads/Drugs/DrugSafety/UCM365078.pdf
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||