Geldanamycin
| Geldanamycin | |
|---|---|
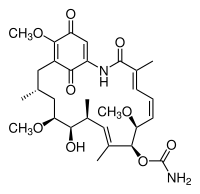 | |
| IUPAC name (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-13-hydroxy | |
| Identifiers | |
| CAS number | 30562-34-6 |
| ChemSpider | 10272739 |
| DrugBank | DB02424 |
| ChEMBL | CHEMBL278315 |
| Jmol-3D images | {{#if:NC(=O)O[C@H]1C(/C)=C/[C@H](C)[C@@H](O)[C@@H](OC)C[C@H](C)C\C2=C(/OC)C(=O)\C=C(\NC(=O)C(\C)=C\C=C/[C@@H]1OC)C2=O|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C29H40N2O9 |
| Molar mass | 560.64 g/mol |
| Appearance | Gold-yellow fine crystalline powder |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Geldanamycin is a benzoquinone ansamycin antibiotic that inhibits the function of Hsp90 (Heat Shock Protein 90) by binding to the unusual ADP/ATP-binding pocket of the protein.[2] HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis.
Geldanamycin induces the degradation of proteins that are mutated in tumor cells such as v-Src, Bcr-Abl and p53 preferentially over their normal cellular counterparts. This effect is mediated via HSP90. Despite its potent antitumor potential, geldanamycin presents several major drawbacks as a drug candidate such as hepatotoxicity, further, Jilani et al. reported that geldanamycin induces eryptosis (apoptosis of erythrocytes) under physiological concentrations.[3] These side effects have led to the development of geldanamycin analogues, in particular analogues containing a derivatisation at the 17 position:- 17-AAG
- 17-DMAG
Biosynthesis
Geldanamycin was originally discovered in the organism Streptomyces hygroscopicus.[4] It is a macrocyclic polyketide that is synthesized by a Type I polyketide synthase. The genes gelA, gelB, and gelC encode for the polyketide synthase. The PKS is first loaded with 3-amino-5-hydroxybenzoic acid (AHBA). It then utilizes malonyl-CoA, methylmalonyl-CoA, and methoxymalonyl-CoA to synthesize the precursor molecule Progeldanamycin.[5] This precursor is subjected to several enzymatic and non-enzymatic tailoring steps to produce the active molecule Geldanamycin, which include hydroxylation, o-methylation, carbamoylation, and oxidation.[6]
Notes
- ↑ Stebbins, C. E.; Russo, A. A.; Schneider, C.; Rosen, N.; Hartl, F. U.; Pavletich, N. P. (1997). "Crystal structure of an Hsp90-geldanamycin complex: Targeting of a protein chaperone by an antitumor agent". Cell 89 (2): 239–250. doi:10.1016/S0092-8674(00)80203-2. PMID 9108479.
- ↑ Schulte, T. W.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensgard, B.; Toft, D.; Neckers, L. M. (1998). "Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin". Cell stress & chaperones 3 (2): 100–108. doi:10.1379/1466-1268(1998)003<0100:ARBTTN>2.3.CO;2. PMC 312953. PMID 9672245.
- ↑ "Geldanamycin-Induced Phosphatidylserine Translocation in the Erythrocyte Membrane". Cell Physiol Biochem 32: 1600–1609. 2013. doi:10.1159/000356596.
- ↑ He, W.; Wu, L.; Gao, Q.; Du, Y.; Wang, Y. (2006). "Identification of AHBA Biosynthetic Genes Related to Geldanamycin Biosynthesis in Streptomyces hygroscopicus 17997". Current Microbiology 52 (3): 197–203. doi:10.1007/s00284-005-0203-y. PMID 16502293.
- ↑ Kim, W.; Lee, D.; Hong, S. S.; Na, Z.; Shin, J. C.; Roh, S. H.; Wu, C. Z.; Choi, O.; Lee, K.; Shen, Y. M.; Paik, S. G.; Lee, J. J.; Hong, Y. S. (2009). "Rational Biosynthetic Engineering for Optimization of Geldanamycin Analogues". ChemBioChem 10 (7): 1243–1251. doi:10.1002/cbic.200800763. PMID 19308924.
- ↑ Lee, D.; Lee, K.; Cai, X. F.; Dat, N. T.; Boovanahalli, S. K.; Lee, M.; Shin, J. C.; Kim, W.; Jeong, J. K.; Lee, J. S.; Lee, C. H.; Lee, J. H.; Hong, Y. S.; Lee, J. J. (2006). "Biosynthesis of the Heat-Shock Protein 90 Inhibitor Geldanamycin: New Insight into the Formation of the Benzoquinone Moiety". ChemBioChem 7 (2): 246–248. doi:10.1002/cbic.200500441. PMID 16381049.
References
- Bedin, M.; Gaben, A. M.; Saucier, C. C.; Mester, J. (2004). "Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest". International Journal of Cancer 109 (5): 643–652. doi:10.1002/ijc.20010. PMID 14999769.
External links
- A comprehensive review about Geldanamycin, 17AAG and 17DMAG
- Geldanamycin from Fermentek
- Geldanamycin bound to proteins in the PDB