Furan
| Furan | |
|---|---|
 |
 |
 |
 |
| IUPAC name Furan | |
| Systematic name Oxole | |
| Other names Furfuran | |
| Identifiers | |
| CAS number | 110-00-9 |
| PubChem | 8029 |
| ChemSpider | 7738 |
| KEGG | C14275 |
| ChEBI | CHEBI:35559 |
| ChEMBL | CHEMBL278980 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C4H4O |
| Molar mass | 68.07 g mol−1 |
| Appearance | Colorless, volatile liquid |
| Density | 0.936 g/mL |
| Melting point | −85.6 °C; −122.1 °F; 187.6 K |
| Boiling point | 31.3 °C; 88.3 °F; 304.4 K |
| Hazards | |
| MSDS | Pennakem |
| R-phrases | R26/27/28, R45 |
| S-phrases | S16, S37, S45, S28 |
| NFPA 704 |
 4
3
1
|
| Flash point | −69 °C; −92 °F; 204 K |
| Autoignition temperature | 390 °C; 734 °F; 663 K |
| Explosive limits | Lower:2.3%, upper:14.3% @ 20 °C |
| LD50 | > 2 g/kg (rat) |
| Related compounds | |
| Related heterocycles | Pyrrole Thiophene |
| Related compounds | Tetrahydrofuran (THF) 2,5-Dimethylfuran Benzofuran Dibenzofuran |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether and acetone, but is slightly soluble in water.[2] It is toxic and may be carcinogenic. Furan is used as a starting point to other specialty chemicals.[3]
History
The name furan comes from the Latin furfur, which means bran.[4] The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limpricht in 1870, although he called it tetraphenol.[5][6]
Production
Industrially, furan is manufactured by the palladium-catalyzed decarbonylation of furfural, or by the copper-catalyzed oxidation of 1,3-butadiene:[3]
In the laboratory, furan can be obtained from furfural by oxidation to furan-2-carboxylic acid, followed by decarboxylation.[7] It can also be prepared directly by thermal decomposition of pentose-containing materials, cellulosic solids especially pine-wood.
Synthesis of furans
The Feist–Benary synthesis is a classic way to synthesize furans, although many syntheses have been developed.[8] One of the simplest synthesis methods for furans is the reaction of 1,4-diketones with phosphorus pentoxide (P2O5) in the Paal–Knorr synthesis. The thiophene formation reaction of 1,4-diketones with Lawesson's reagent also forms furans as side products. Many routes exist for the synthesis of substituted furans.[9]
Chemistry
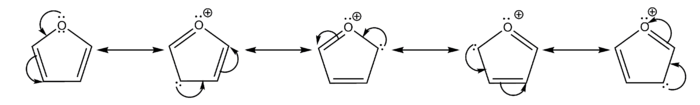
Furan is aromatic because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n+2 aromatic system (see Hückel's rule) similar to benzene. Because of the aromaticity, the molecule is flat and lacks discrete double bonds. The other lone pair of electrons of the oxygen atom extends in the plane of the flat ring system. The sp2 hybridization is to allow one of the lone pairs of oxygen to reside in a p orbital and thus allow it to interact within the pi-system.
Due to its aromaticity, furan's behavior is quite dissimilar to that of the more typical heterocyclic ethers such as tetrahydrofuran.
- It is considerably more reactive than benzene in electrophilic substitution reactions, due to the electron-donating effects of the oxygen heteroatom. Examination of the resonance contributors shows the increased electron density of the ring, leading to increased rates of electrophilic substitution.[10]
- Furan serves as a diene in Diels-Alder reactions with electron-deficient dienophiles such as ethyl (E)-3-nitroacrylate.[11] The reaction product is a mixture of isomers with preference for the endo isomer:
- Hydrogenation of furans affords sequentially dihydrofurans and tetrahydrofurans.
- In the Achmatowicz reaction, furans converted to dihydropyran compounds.
Safety
Furan is found in heat-treated commercial foods and it is produced through thermal degradation of natural food constituents.[12][13] Notably, it can be found in roasted coffee, instant coffee, and processed baby foods.[14][13][15] Exposure to furan at doses about 2000 times the projected level of human exposure from foods increases the risk of hepatocellular tumors in rats and mice and bile duct tumors in rats.[16] Furan is therefore listed as a possible human carcinogen.[16]
See also
- BS 4994 – Furan resin as thermoset FRP for chemical process plant equipments
- Furanoflavonoid
- Furanose
- Furantetracarboxylic acid
- Simple aromatic rings
References
- ↑ Webster's Online Dictionary
- ↑ Hans Dieter Jakubke; Hans Jeschkeit (1994). Concise Encyclopedia of Chemistry. Walter de Gruyter. pp. 001–1201. ISBN 0-89925-457-8.
- ↑ 3.0 3.1 H. E. Hoydonckx, W. M. Van Rhijn, W. Van Rhijn, D. E. De Vos, P. A. Jacobs (2005), "Furfural and Derivatives", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a12_119.pub2
- ↑ Alexander Senning. Elsevier's Dictionary of Chemoetymology. Elsevier, 2006. ISBN 0-444-52239-5.
- ↑ Limpricht, H. (1870). "Ueber das Tetraphenol C4H4O". Berichte der deutschen chemischen Gesellschaft 3 (1): pp. 90–91. doi:10.1002/cber.18700030129.
- ↑ Rodd, Ernest Harry (1971). Chemistry of Carbon Compounds: A Modern Comprehensive Treatise. Elsevier.
- ↑ Wilson, W.C. (1941), "Furan", Org. Synth.; Coll. Vol. 1: 274
- ↑ Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HNC (1998). "Regioselective syntheses of substituted furans". Tetrahedron 54 (10): 1955–2020. doi:10.1016/S0040-4020(97)10303-9.
- ↑ Katritzky, Alan R. (2003). "Synthesis of 2,4-disubstituted furans and 4,6-diaryl-substituted 2,3-benzo-1,3a,6a-triazapentalenes". Arkivoc 2004 (2): 109. doi:10.3998/ark.5550190.0005.208.
- ↑ Bruice, Paula Y. (2007). Organic Chemistry (Fifth ed.). Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 0-13-196316-3.
- ↑ Masesane I, Batsanov A, Howard J, Modal R, Steel P (2006). "The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acid". Beilstein Journal of Organic Chemistry 2 (9): 9. doi:10.1186/1860-5397-2-9. PMC 1524792. PMID 16674802.
- ↑ Anese, M; Manzocco, L; Calligaris, S; Nicoli, MC (2013). "Industrially Applicable Strategies for Mitigating Acrylamide, Furan and 5-Hydroxymethylfurfural in Food". Journal of agricultural and food chemistry: 130528102950009. doi:10.1021/jf305085r. PMID 23627283.
- ↑ 13.0 13.1 Moro, S; Chipman, JK; Wegener, JW; Hamberger, C; Dekant, W; Mally, A (2012). "Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment". Molecular nutrition & food research 56 (8): 1197–211. doi:10.1002/mnfr.201200093. PMID 22641279.
- ↑ European Food Safety Authority (2011). EFSA Journal 9 (9): 2347. doi:10.2903/j.efsa.2011.2347.
- ↑ Waizenegger, J; Winkler, G; Kuballa, T; Ruge, W; Kersting, M; Alexy, U; Lachenmeier, DW (2012). "Analysis and risk assessment of furan in coffee products targeted to adolescents". Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment 29 (1): 19–28. doi:10.1080/19440049.2011.617012. PMID 22035212.
- ↑ 16.0 16.1 Bakhiya, N; Appel, KE (2010). "Toxicity and carcinogenicity of furan in human diet". Archives of toxicology 84 (7): 563–78. doi:10.1007/s00204-010-0531-y. PMID 20237914.

